New OCR Gateway specification from
September 2016 Higher tier: grades 9 to 4:
In this and subsequent posts I’m simply going to explain and illustrate each learning objective as they come up in the topics in the new GCSE specification.
I’m giving you my notes from each lesson.
You can really get ahead of your class if you follow this blog and all the posts that will appear here about the new GCSEs over the coming months.
This rejigging of the specification is just that: there is nothing really new here it has all been with us for the past half century at least.
That written in italics is for the higher tier paper only.
C3 Chemical Reactions
C3.1 Introducing chemical reactions
In this and subsequent posts I’m simply going to explain and illustrate each learning objective as they come up in the topics in the new GCSE specification.
I’m giving you my notes from each lesson.
You can really get ahead of your class if you follow this blog and all the posts that will appear here about the new GCSEs over the coming months.
This rejigging of the specification is just that: there is nothing really new here it has all been with us for the past half century at least.
That written in italics is for the higher tier paper only.
C3 Chemical Reactions
C3.1 Introducing chemical reactions
Chemical Equations and the Mole
C3.1g recall and
use the definitions of the Avogadro constant (in standard form) and of the mole
and the calculation of the mass of one atom/molecule
The Avogadro Constant is a number like the number in a
dozen, 12 or in a score, 20 or in a gross, 144 or in a ream, 500.
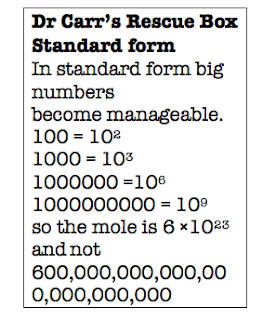
A mole of particles or anything else is 6.02 × 1023
in standard form.
Written out in full that’s 602,000,000,000,000,000,000,000.0
And a mole of atoms will have mass.
12.0000g of the isotope carbon C12 contains
exactly one mole of atoms and is the standard atomic mass.
The relative atomic mass of an element in units of
grams contains exactly one mole of particles.
So 12.0000g carbon contains one mole of atoms.
23g of sodium metal contains one mole of sodium atoms.
But 32.00g of oxygen gas contains one mole of oxygen
molecules O2.
That’s because oxygen gas exists as diatomic molecules
O2 each with a RAM of 16.
So 124g of phosphorus contains one mole of phosphorus
molecules P4.
That’s because phosphorus
can exist as P4 molecules and each atom has a RAM of 31.
It works the same for
molecules.
So 18g of water contains one
mole of water molecules (H2O) since the RAM of oxygen is 16 and that
of hydrogen 1.0.
So two moles of water will
contain 2 × 6 ×1023 molecules.
And two moles will have a
mass of 2 × 18g = 36g
C3.1h explain how the mass of a given substance is
related to the amount of that substance in moles and vice versa
So from this we can say that
amt of substance (moles) ×
RMM(g/mol) = mass of substance (g)
The relationship between
mass of substance m (in grams), amount of substance n (in moles) and RAM or RMM
M (in g/mol) is this:
n =
m/M
in words:
amount of substance n
(moles) = mass of substance m (grams)
RMM M (g/mol)
Problems:
1.
How many moles of chlorine
in 71g?
2.
How many moles of neon in
40g?
3.
How many moles of sodium in
69g?
4.
How many moles of water in
72g?
5.
How many moles of ethanol (C2H5OH)
in 92g?
6.
What mass is there in 6
moles of sulphur S8?
7.
What mass is there in 10
moles of sodium carbonate (Na2CO3)?
8.
What mass is there in 1.5
moles of water?
9.
What mass is there in 4.67
moles of sugar (C12H22O11)?
10. What mass is there in 7.2 moles of sodium chloride (NaCl)?
Using the mole to calculate
masses of reactants and products and to create balanced symbol equations.
C3.1k deduce the stoichiometry of an equation from the
masses of reactants and products and explain the effect of a limiting quantity
of a reactant.
Stoichiometry means the mole
ratio of reacting substances in a chemical equation and the mole ratio of the
products formed.
In this equation the
stoichiometry is that two moles of hydrogen react with one mole of oxygen to
form two moles of water.
2H2(g) + O2(g) =
2H2O(l)
If we know that 18g of
aluminium reduce exactly 53.3g of iron(III)oxide to form 37.3g of iron and 34g
of aluminium oxide then we can work out the stoichiometry of the equation.
Method:
1. Word
equation
|
Aluminium
+ iron oxide = iron +
aluminium oxide
|
2. Masses
(g)
|
18 53.3 37.3 34
|
3. Molar
masses (g/mol)
|
27 160 56 102
|
4. Moles
(2./3.) i.e. use n=m/M
|
0.67 0.33 0.66 0.33
|
5. Simplest
mole ratio
|
2 1 2 1
|
6. Final
equation
|
2Al +
Fe2O3
= 2Fe + Al2O3
|
Now if we
had used not 18g of aluminium but 30g there would not have been enough iron
oxide to react with it.
The iron
(III) oxide is the limiting reactant.
There would
have been 12g of aluminium left over contaminating the iron formed.
So a
limiting reactant means some reaction cannot take place because reactions have
to follow the stoichiometry rules as in the example above where one mole of
iron(III)oxide (no more no less) reacts exactly with two moles of aluminium.
The
stoichiometry of a reaction states exactly how many moles of the two reactants
will combine together.
C3.1l use a balanced equation to calculate masses of
reactants or products.
How to find the mass of
product that forms in a reaction.
How much iron forms when
carbon monoxide reduces 174g of iron(III)oxide in a Blast Furnace?
Method:
Word equation
|
Iron(III) oxide + carbon monoxide = iron + carbon dioxide
|
Symbols
|
Fe2O3 + 3CO = 2Fe + 3CO2
|
Reacting masses (g)
|
160 84 112 132
|
Calculation
|
|
Answer
|
121.8g of iron
|
C3.1i recall and use the law
of conservation of mass
The law of conservation of mass states that
matter is neither created nor destroyed in a chemical change.
So the total mass of the reactants must be the
same as the total mass of the products in a chemical change.
Example
2Al + Fe2O3 = 2Fe + Al2O3
54g 160g = 112g 102g
total mass reactants 214g total mass products 214g
This principle is true for every chemical change
C3.1j explain any observed
changes in mass in non-enclosed systems during a chemical reaction and explain
them using the particle model.
So when the mass of reactants does not equal the
mass of product it is likely that a gas was given off
Example:
Mg(s) + 2HCl(aq) = MgCl2(aq) + H2(g)
24g 73g ≠ 95g
mass of reactants mass
of observed products
The missing 2g is the hydrogen gas that escapes
into the atmosphere.










No comments:
Post a Comment