What are Geometric Isomers in alkenes and how do they arise?

This last example shows how the geometric isomerism can exist around other structures not just the carbon carbon double bond.
So what happens to cis and trans designations when you have this kind of a substituted alkene?
With more complex alkenes, the designations E (for the German Entgegen) and Z (for the German Zusamen) are used to distinguish the isomers.
This is how it works:
This compound is (E) 1-bromo-2-chloro-2-fluoroethene.
The different ends of the molecule are treated separately.
The two groups on the end of the double bond are compared to see which has the higher atomic number.
In the example above, on the left bromine has the higher atomic number and on the right chlorine has the higher atomic number.
Atoms with the higher atomic number have what is called priority.
If higher priority atoms (or groups) are cis to each other that is the Z isomer.
In the above example the higher priority atoms are trans to each other so that is the E isomer.
If there are just alkyl groups on the ends of the double bond then as a general rule the group with the higher number of carbons has priority.
See the example below where on the left the CH3— has priority over H–— and on the right (CH3)2CH— has priority over CH3CH2— putting higher priority groups on the same side therefore it is the Z isomer:
Let's go back the the basic alkene structure of ethene C2H4.
The carbon carbon double bond composed of the σ and the π bond cannot rotate easily.
To rotate the σ bond, the π bond would have to break requiring some input of energy.
So if there are different groups attached to the double bond carbons, different structures fit the same molecular formula.
We have what are called geometric isomers.
A common example quoted is the literature is but-2-ene C4H8.
You can find a short (less than 2mins) tutorial here on geometric isomerism though you might be a tad frustrated with the fact that its a preview version — still better than paying I say.
Here are the two geometric isomers of but-2-ene:
So the condition for geometric isomerism is that there are two different groups attached to the Carbon atoms of the double bond.
In this case a methyl group (CH3) and a hydrogen atom.
Trans refers to the isomer where the groups are on different sides of the double bond.
Trans is Latin for "across", i.e. the groups are across from one another.
Cis refers to the isomer where the groups are on the same side of the double bond.
Other examples are:

This last example shows how the geometric isomerism can exist around other structures not just the carbon carbon double bond.
So what happens to cis and trans designations when you have this kind of a substituted alkene?
With more complex alkenes, the designations E (for the German Entgegen) and Z (for the German Zusamen) are used to distinguish the isomers.
This is how it works:
This compound is (E) 1-bromo-2-chloro-2-fluoroethene.
The different ends of the molecule are treated separately.
The two groups on the end of the double bond are compared to see which has the higher atomic number.
In the example above, on the left bromine has the higher atomic number and on the right chlorine has the higher atomic number.
Atoms with the higher atomic number have what is called priority.
If higher priority atoms (or groups) are cis to each other that is the Z isomer.
In the above example the higher priority atoms are trans to each other so that is the E isomer.
If there are just alkyl groups on the ends of the double bond then as a general rule the group with the higher number of carbons has priority.
See the example below where on the left the CH3— has priority over H–— and on the right (CH3)2CH— has priority over CH3CH2— putting higher priority groups on the same side therefore it is the Z isomer:







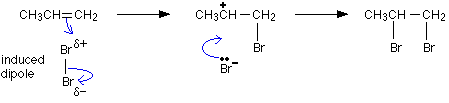





















.gif)




















