Edexcel A
level Chemistry (2017)
Topic 10:
Equilibrium I:
Here is
the fifth learning objective:
10/I/4. To
be able to deduce an expression for Kc , for homogeneous and heterogeneous
systems, in terms of equilibrium concentrations
There is a mathematical model that fits an equilibrium reaction.
If wA +
xB ⇋ yC
+ zD then
Kc is called the equilibrium constant.
It is constant at constant temperature.
And [C]y is the equilibrium concentration of species C
in the chemical equation raised to the power of its stoichiometric coefficient
y.
Ammonia and the Haber process
The overall reaction vessel equation is:
N2
(g) + 3H2 (g) ⇌ 2NH3 (g) ΔH = –92kJ/mol
where eqm refers to the
equilibrium concentrations of each species in the equilibrium.
The units of the equilibrium constant are:
which leaves the units of the equilibrium constant as
so the units of Kc change according to the chemical change
involved.
Example 2:
A aqueous homogenous equilibrium
Esterification:
CH3CH2OH(l) + CH3COOH(l) ⇌ CH3COOCH2CH3(l) + H2O(l)
Here Kc has no units.
Example 3: A
heterogeneous equilibrium.
A heterogeneous reaction is one in which
the reactants and/or products are not all in the same physical state at room
temperature and pressure.
CaCO3
(s) ⟶
CaO(s) + CO2(g)
In
this example, the solids do not have a vapour pressure or a concentration so they
cannot be included in the equilibrium expression.
Therefore: Kc =
[CO2(g)]eqm
Kc
has units of mol.dm-3
There
are Labs available to use to calculate experimentally a value of an equilibrium
constant.
Measuring an
equilibrium constant Kc
AS the specification for the Edexcel A level suggests
“Possible experiments include investigating equilibrium systems, such as
iron(III) – thiocyanate,….”
In
this experiment, the slow redox reaction between silver ions and iron(III) ions
is studied.
The
equilibrium is convenient to study because it establishes itself reasonably
quickly i.e. over a few days and we can get at its value using a precipitation
titration.
Ag+
(aq) + Fe 2+ (aq) ⇌ Ag(s) + Fe 3+ (aq)
Samples
of the resulting equilibrium mixture containing silver ions are titrated with potassium
thiocyanate solution (KCNS).
The
titration results are used to calculate Kc from the expression:
The
reaction between silver ions and potassium thiocyanate is at first a
precipitation of insoluble silver thiocyanate (AgCNS)
KCNS(aq) +
AgNO3 (aq) ⟶ KNO3(aq) +
AgCNS
But when all the silver ions have
been precipitated from the equilibrium mixture the thiocyante ions react with
iron(III) ions in the resultant equilibrium mixture to give the deep red colour
of the iron(III) thiocyanate complex.
Fe3+ +
CNS– ⟶ Fe(CNS)2+
We assume that the equilibrium cannot
re-establish itself fast enough in the time we do the titration.
A Practical Script
Preparation:
• Place 25ml of a solution of 0.10M
silver nitrate in a dry 100ml conical flask.
• Add 25ml of a solution of 0.10M
iron(II) sulphate to the same flask.
• Stopper and shake the flask and
leave to stand overnight.
Practical:
• After equilibrium has been
established, use a pipette to transfer 10.0ml of the solution to another
conical flask. Ensure that the pipette
does not pick up or disturb the silver precipitate in the flask.
• Titrate the 10ml sample of the
equilibrium mixture with 0.020M potassium thiocyanate.
• Look for the end point when the
first permanent red colour is seen in the conical flask.
• It should be possible for you to
repeat the titration twice.
• Calculate the average titre from
the “best” of your results.
• Calculate the concentration of free
silver ions at equilibrium from your titration results and call this value x.
Calculation
of Kc:
At
equilibrium [Ag+(aq)]eqm = [Fe2+(aq)]eqm
= x
And
[Fe3+(aq)] eqm = [Fe2+(aq)]
initial – x i.e.
0.05 –
x
Fill
in this table:
[Fe2+(aq)]
|
[Ag+(aq)]
|
Ag(s)
|
[Fe3+(aq)]
|
|
Initial
concentrations
|
0.05
|
0.05
|
0.00
|
0.00
|
Equilibrium
concentrations
|
x
|
x
|
0.05–x
|
And calculate Kc
(in 2007 I calculated Kc to be 55 mol-1.dm3 at 21oC)
Questions:
1.
Why is Ag(s) irrelevant to your calculation?
2.
Why are the units of Kc mol-1.dm3
?
3.
Why leave the reaction mixture overnight and why must the flask be sealed with
a stopper?



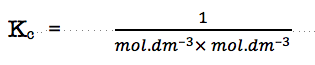












No comments:
Post a Comment