New OCR Gateway specification from September
2016 Higher tier: grades 9 to 4:
C1.1 The Particle Model:
In this and subsequent posts I’m simply going to explain and illustrate each learning objective as they come up in the topics in the new GCSE specification.
You can really get ahead of your class if you follow this blog and all the posts that will appear here about the new GCSEs over the coming months.
This rejigging of the specification is just that: there is nothing really new here since “states of matter” and the particle model has been with us for the past half century at least.
That written in italics is for the higher tier paper only.
C1.1a describe the main features of the particle model in terms of states of matter and change of state
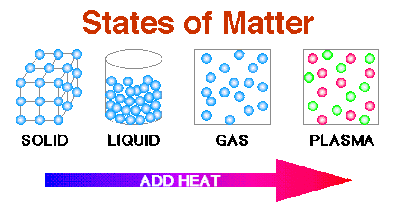
There three states of matter: solid, liquid and gas.
Plasma occurs at a temperature where the atoms of the material have been stripped of electrons.
What makes each state different from the other and how does a substance move from one state to another?
The Solid State:
At this point we make a massive assumption in chemistry which is that all substances are particulate i.e. they consist of small particles so small that even the most advanced microscopes cannot “see’ them but only see the effects of their presence as the constituent particles of a substance.
There three types of particle:
Atom: the smallest part of any element.
Elements consist of only one type of atom.
So iron consists of iron atoms only. I know it sounds obvious but someone has to say it!!
Molecule: the smallest particle in a compound.
Molecules consist of more than one type of atom. They are simply groups of atoms chemically bonded to each other. Most substances are made up of molecules like water H2O or DNA.
Ion: an ion is an electrically charged atom or molecule.
There two types of ion: positively charged ions or cations and negatively charged ions or anions. Oppositely charged ions attract each other electrostatically.
Did you know that Michael Faraday coined the word ion for these particles when he first demonstrated electrolysis in the early 19th century.
So whether stuff is made up of atoms or molecules or ions they behave in the same way in the solid state.
In iron (atoms) or water (molecules) or salt (ions) the particles at room temperature are arranged in a regular pattern (as in the picture below) as close together as they can get and the particles themselves move by vibrating about a fixed point.
The Liquid State:
Raise the temperature of the solid and eventually it will begin melt.
It will change state into a liquid.
The melting point has been reached. At this temperature, the energy given to the solid does not warm up the solid but instead it works to overcome the forces of attraction between its particles.
Once all the forces of attraction have been overcome the solid has fully changed into a liquid.
The particles are no longer arranged in a regular 3D pattern but now are randomly ordered. There is no greater distance between the particles in a liquid than there was in the solid but they can now move in straight lines.
They slip and slide and roll over and around each other (see the picture below) . The particles are constantly in motion. The volume of the liquid is much the same as the solid. And just as you cannot compress a solid you can’t compress a liquid either.
Think of a packed school disco and all the teenage sweaty bodies squirming on the dance floor very close together moving around each other to the music. Bodies equals particles…..on second thoughts perhaps don’t think of that image: Ugh!!!
The Gaseous State:
Raise the temperature of the liquid and eventually it will begin to boil.
It will change state into a gas.
The boiling point has been reached. At this temperature the energy given to the liquid does not warm up the liquid but instead it works to overcome the forces of attraction that still exist between the particles. Also the vapour pressure of the liquid equals the atmospheric pressure and so the liquid boils.
Once all the forces of attraction have been overcome the liquid has fully changed into a gas.
The particles are now flying apart moving in straight lines until they collide with another particle.
The substance has expanded and now fills the container it is in.
The particles are very far apart.
Between collisions they move at incredibly fast speeds.
Their movement is unpredictable and random.
The substance exerts a pressure in its container as the particles collide with and bounce off the walls.
And this gas has now become compressible because of the big spaces between the particles.
To picture this, think of many wasps (wasps equals particles) very annoyed because they are trapped in a jam jar, only they never stop for a rest unlike real wasps.
Here are some images and descriptions of states of matter you can find on the internet all of which have their faults; can you spot them?
C1.1b explain in terms of the particle model the distinction between physical changes and chemical changes
Physical changes:
Essentially physical changes involve changes of the position and speed of the particles of a substance.
Physical changes are changes of state:
Freezing in which a liquid turns into a solid at constant temperature.
Boiling in which a liquid turns into a gas at constant temperature.
Condensation in which a gas turns into a liquid at constant temperature.
Melting in which a solid turns into a liquid at constant temperature.
Sublimation in which a solid turns directly into a gas or vice versa at constant temperature.
Chemical Changes:
Chemical changes are different from Physical changes because in chemical changes the actual arrangement and bonds between atoms or ions change to produce new substances.
Example:
Hydrogen burning in air:
2H2(g) + O2(g) = 2H2O(l)
The bonds between the hydrogen atoms and the oxygen atoms must break for the new bonds between hydrogen and oxygen to form in water.
In the diagram hydrogen atoms are in white and oxygen atoms are in red.
C1.1c explain the limitations of the particle model in relation to changes of state when particles are represented by inelastic spheres (e.g. like bowling balls)
that it does not take into account the forces of attraction between particles, the size of particles and the space between them.
The problem with any particle model is that it cannot take into account the fact that particles are fields of electrical charge, electrons spinning around a small nucleus of protons and neutrons, as this diagram attempts to show:
The circles/sphere representing atoms/ions/molecules on paper or in physical models where beads or billiard balls are used are just too hard and inflexible to be a good representation.
All particles have significant forces of attraction between them and that is not true of any of the “particles” in a model.
All particles are in constant motion all the time: motion only stops at absolute zero, i.e. zero degrees Kelvin or –273oC.
All particles take up space themselves so that in a gas for example the volume they occupy is different from the volume of the gas itself. The space between the particles is less that the space they actually occupy as a gas.
WS1.1c Observations of change of state with comparison to chemical changes
C1.1 The Particle Model:
In this and subsequent posts I’m simply going to explain and illustrate each learning objective as they come up in the topics in the new GCSE specification.
You can really get ahead of your class if you follow this blog and all the posts that will appear here about the new GCSEs over the coming months.
This rejigging of the specification is just that: there is nothing really new here since “states of matter” and the particle model has been with us for the past half century at least.
That written in italics is for the higher tier paper only.
C1.1a describe the main features of the particle model in terms of states of matter and change of state
There three states of matter: solid, liquid and gas.
Plasma occurs at a temperature where the atoms of the material have been stripped of electrons.
What makes each state different from the other and how does a substance move from one state to another?
The Solid State:
At this point we make a massive assumption in chemistry which is that all substances are particulate i.e. they consist of small particles so small that even the most advanced microscopes cannot “see’ them but only see the effects of their presence as the constituent particles of a substance.
There three types of particle:
Atom: the smallest part of any element.
Elements consist of only one type of atom.
So iron consists of iron atoms only. I know it sounds obvious but someone has to say it!!
Molecule: the smallest particle in a compound.
Molecules consist of more than one type of atom. They are simply groups of atoms chemically bonded to each other. Most substances are made up of molecules like water H2O or DNA.
Ion: an ion is an electrically charged atom or molecule.
There two types of ion: positively charged ions or cations and negatively charged ions or anions. Oppositely charged ions attract each other electrostatically.
Did you know that Michael Faraday coined the word ion for these particles when he first demonstrated electrolysis in the early 19th century.
So whether stuff is made up of atoms or molecules or ions they behave in the same way in the solid state.
In iron (atoms) or water (molecules) or salt (ions) the particles at room temperature are arranged in a regular pattern (as in the picture below) as close together as they can get and the particles themselves move by vibrating about a fixed point.
The Liquid State:
Raise the temperature of the solid and eventually it will begin melt.
It will change state into a liquid.
The melting point has been reached. At this temperature, the energy given to the solid does not warm up the solid but instead it works to overcome the forces of attraction between its particles.
Once all the forces of attraction have been overcome the solid has fully changed into a liquid.
The particles are no longer arranged in a regular 3D pattern but now are randomly ordered. There is no greater distance between the particles in a liquid than there was in the solid but they can now move in straight lines.
They slip and slide and roll over and around each other (see the picture below) . The particles are constantly in motion. The volume of the liquid is much the same as the solid. And just as you cannot compress a solid you can’t compress a liquid either.
Think of a packed school disco and all the teenage sweaty bodies squirming on the dance floor very close together moving around each other to the music. Bodies equals particles…..on second thoughts perhaps don’t think of that image: Ugh!!!
The Gaseous State:
Raise the temperature of the liquid and eventually it will begin to boil.
It will change state into a gas.
The boiling point has been reached. At this temperature the energy given to the liquid does not warm up the liquid but instead it works to overcome the forces of attraction that still exist between the particles. Also the vapour pressure of the liquid equals the atmospheric pressure and so the liquid boils.
Once all the forces of attraction have been overcome the liquid has fully changed into a gas.
The particles are now flying apart moving in straight lines until they collide with another particle.
The substance has expanded and now fills the container it is in.
The particles are very far apart.
Between collisions they move at incredibly fast speeds.
Their movement is unpredictable and random.
The substance exerts a pressure in its container as the particles collide with and bounce off the walls.
And this gas has now become compressible because of the big spaces between the particles.
To picture this, think of many wasps (wasps equals particles) very annoyed because they are trapped in a jam jar, only they never stop for a rest unlike real wasps.
Here are some images and descriptions of states of matter you can find on the internet all of which have their faults; can you spot them?
C1.1b explain in terms of the particle model the distinction between physical changes and chemical changes
Physical changes:
Essentially physical changes involve changes of the position and speed of the particles of a substance.
Physical changes are changes of state:
Freezing in which a liquid turns into a solid at constant temperature.
Boiling in which a liquid turns into a gas at constant temperature.
Condensation in which a gas turns into a liquid at constant temperature.
Melting in which a solid turns into a liquid at constant temperature.
Sublimation in which a solid turns directly into a gas or vice versa at constant temperature.
Chemical Changes:
Chemical changes are different from Physical changes because in chemical changes the actual arrangement and bonds between atoms or ions change to produce new substances.
Example:
Hydrogen burning in air:
2H2(g) + O2(g) = 2H2O(l)
The bonds between the hydrogen atoms and the oxygen atoms must break for the new bonds between hydrogen and oxygen to form in water.
In the diagram hydrogen atoms are in white and oxygen atoms are in red.
C1.1c explain the limitations of the particle model in relation to changes of state when particles are represented by inelastic spheres (e.g. like bowling balls)
that it does not take into account the forces of attraction between particles, the size of particles and the space between them.
The problem with any particle model is that it cannot take into account the fact that particles are fields of electrical charge, electrons spinning around a small nucleus of protons and neutrons, as this diagram attempts to show:
The circles/sphere representing atoms/ions/molecules on paper or in physical models where beads or billiard balls are used are just too hard and inflexible to be a good representation.
All particles have significant forces of attraction between them and that is not true of any of the “particles” in a model.
All particles are in constant motion all the time: motion only stops at absolute zero, i.e. zero degrees Kelvin or –273oC.
All particles take up space themselves so that in a gas for example the volume they occupy is different from the volume of the gas itself. The space between the particles is less that the space they actually occupy as a gas.
WS1.1c Observations of change of state with comparison to chemical changes
The
classic experiment on change of state to measure the melting/freezing point of
a substance is that using Stearic acid.
The
experiment can be found on Youtube:



















No comments:
Post a Comment