#2 Nucleophilic
substitution in primary
In a previous post I discussed the basics of this reaction
between haloalkanes and aqueous alkali.
Here is the general reaction scheme:
We can make some criticisms of this image from the web:
The curly arrows are pretty random and sloppily drawn.
We’d like to see them start on a lone pair of the
nucleophile and end on a specific positive centre.
We’d also like to see them start on the R—X bond and end as
they do on the halide lone pair.
These generic mechanisms do not indicate anything about the
rate at which these reactions proceed.
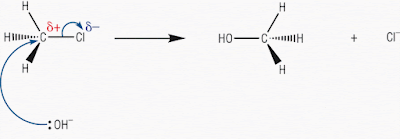
Let’s look at another representation of this reaction
mechanism from the web:
Now this is the hydrolysis of chloromethane: a primary haloalkane
Note how the arrows are drawn, where they start and where
they end: brilliant, copy this!!
This reaction proceeds in one flowing movement.
The only thing missing is the intermediate transition state.
But this mechanism does show the way in which the geometry of the primary haloalkane
‘flips over’.
There will be more about the consequences of this ‘flip
over’ in a later post.
The reaction involves both
the haloalkane and the hydroxide ion in the slowest or rate determining step i.e. it is bimolecular.
The reaction is substitution
(S), nucleophilic(N) and bimolecular(2)
That’s why it is called an Sn2 reaction!!
Of course the orientation of the attacking group has to be
right too.
The stereo chemistry of this reaction is important.
The hydroxide ion attacks from the opposite side to the
leaving group the chloride (halide) ion.
So what about the intermediate transition state?
Let’s look at another image from the web (from chemguide) to
show this transition state:
The thing here is to see that the transition state is negatively charged
We write the transition
state in square brackets.
I prefer to see curly
arrows in red, unless it is on an exam paper, there’s a long historic
reason for me saying that that I shall talk about on this blog sometime.
The other striking geometry is that the transition state has five covalent bonds and the central structure of it is planar.
What is the CH3-C-H bond angle in the transition
state?
Two of the bonds in the transition state are partial, one forming as one breaks,
hence the use of dotted lines to
show this feature.
The transition state then is a temporary structure with a half life of around 10—12
seconds.
Primary haloalkanes hydrolyse by this mechanism because
there are insufficient methyl groups on the primary carbon to stabilise a
carbocation as an intermediate structure.
In my next post, I’ll discuss hydrolysis of tertiary halogenoalkanes











No comments:
Post a Comment