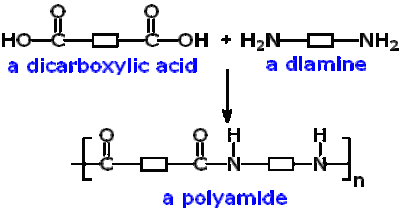
C6.2e To be able to explain the basic principles of condensation
polymerisation
With reference to the functional groups of the monomers, the minimum number
of functional groups within a monomer, the number of repeating units in the
polymer, and simultaneous formation of a small molecule, e.g. a polyester or
polyamide, using block diagrams to represent polymers
C6.2f To be able to describe practical techniques to make a polymer by
condensation
This content is only a higher tier requirement.
Condensation polymerisation to form nylon
Nylon was
one of the first synthetic polymers to be produced. Carothers first formed nylon in the mid
1930’s. It is said that the name nylon
is the combination of two places New
York and London—I’ll leave you to check that out for yourself.
Nylon is pretty
indestructible and takes years to degrade in the ground and that’s why it last
for even longer in the sea. It is the
cause of major sea pollution and hazardous to sea life.
All nylon is
made from precursor molecules from crude oil.
But some things really ought not to be made from it when they are easily
thrown away and then do not rot down.
The use of
nylon ought to be reserved for specialist applications.
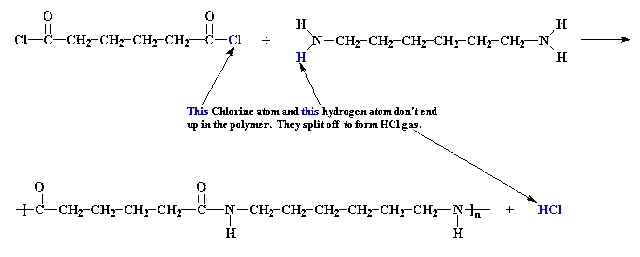
Here is what
happens in the reaction between these two compounds:
NH2(CH2)6NH2
+ ClOC(CH2)4COCl ➞ H[HN(CH2)6NHCO(CH2)4CO]nCl + HCl
The two
molecules add together and lose a small molecule of HCl.
Addition
followed by the elimination of a small molecule adds up to a condensation
reaction.
The number
of carbon atoms in the monomers determines the name of the nylon. So in this case the nylon is nylon 6:6
because each monomer contains six carbon atoms.
Note how
there are square brackets around part of the polymer structure. This is the repeat unit of the polymer and n
is the number of repeat units involved on average in the polymer sample and
e.g. it could be 500-1000.
It’s easy to
place the brackets because outside them should be the atoms that form the small
molecule eliminated from the two monomers.
Note that
there are two functional groups in each monomer.
Preparing
nylon 6:6 in the lab is fairly straightforward.
You need the two monomers in the appropriate solutions, the amine in
alkaline sodium carbonate solution and the acid chloride in an organic solvent
such as hexane.
Pour the
amine solution into a small 100ml beaker and then carefully pour the hexane
solution of the other monomer over the top.
Hexane having the lower density should float on the aqueous solution.
At the
interface of the two solutions the polymer will form spontaneously and using
tweezers you should be able to pull a continuous thread of nylon from there
until the solutions are exhausted of monomer.
Here are a
couple of pictures to illustrate this procedure one of which is more outrageous
than the other!!












No comments:
Post a Comment