Edexcel A level
Chemistry (2017)
Topic 15A:
Principles of transition metal chemistry
Using the Beer-Lambert Law to measure the unknown
concentration of a transition metal ion in solution.
Colorimetry is used to measure the concentration of a coloured ion in
solution.
In Colorimetry, we measure how much visible
light a coloured solution of a given concentration is absorbing.
The measurements are taken using a colorimeter. (see diagram)
The light passes through a filter. The filter selects the wavelength
of light closest to maximum absorption. The coloured solution (in a cuvette) absorbs some of this
light.
The light absorbed is called the absorbance. The greater the concentration of coloured
ions, the greater the absorbance.
In less expensive colorimeters like those you have in school, you first
zero the instrument using a cuvette of 'colorless' water.
This sets readings to zero absorbance.
Next you put in a cuvette of the coloured solution and read off the
'absorbance'.
‘Zeroing' eliminates error because even a 'colorless blank' of cuvette and
water can absorb a tiny amount of light.
How you can use colorimetry to measure the
concentration of a transition metal ion in solution.
You will need to create a calibration curve of absorbance vs standard concentrations
and use it to measure the concentration of an unknown solution of your
transition metal ion.
The absorbance of an intensely coloured transition metal ion in aqueous
solution can be measured directly e.g. the concentration of manganese in the
deep purple manganate(VII) ion, MnO4—.
But the absorbances of much less intensely coloured solutions of transition
metals are more difficult to measure e.g. light blue hexaaquacopper(II) ions.
So the thing to do is to create an intensely coloured solution using ligand
exchange. The intensely coloured solution then gives accurate measures of absorbance
that can then be turned into accurate measures of concentration.
So add ammonia solution to the blue hexaaquacopper(II) ion to form the deep
blue ammine complex.
Or add potassium thiocyanate to a brown solution of iron(III) ions to form
the deep blood–red thiocyanate ion (SCN–) complex.
Creating the calibration curve
To do this set up a range of
concentrations of your transition metal ion solution. Next you have to measure the absorbance of
each of these solutions, preferably more than once, to establish a reliable
calibration curve.
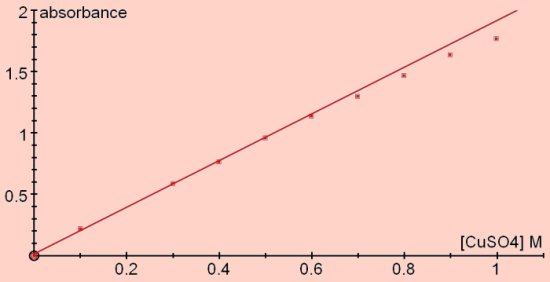
Next plot your results (see
below) This plot shows results for the absorbance of different concentrations
of copper (II) ions.
Providing you work at
reasonably low concentrations (probabaly <1.0M), you will find that the
calibration curve is a straight line (as above). In
other words, it obeys a law, the Beer—Lambert Law: as we said above, the
greater the concentration, the greater the absorbance.
Absorbance is directly
proportional to the concentration of the aqueous solution.
All you have to do now is take
your solution of transition metal ion whose concentration you don’t know,
measure its absorbance and read off the concentration from the calibration
curve.










No comments:
Post a Comment