Edexcel A level Chemistry (2017)
Topic 14: Redox(II): Fuel Cells
Here are the learning objectives relating to
fuel cells:
14/16. To be able to understand that the energy released on the reaction of
a fuel with oxygen is utilized in a fuel cell to generate a voltage.
Knowledge that
methanol and other hydrogen-rich fuels are used in fuel cells is expected.
14/17. To know the electrode reactions that occur in a hydrogen-oxygen fuel
cell.
Knowledge of
hydrogen-oxygen fuel cells with both acidic and alkaline electrolytes is
expected.
The Proton
Exchange Fuel Cell
Proton exchange membrane fuel cells are a
type of fuel cell being developed to replace conventional alkaline fuel cells
of the type used in the Space Shuttle.
Their distinguishing features include lower
temperature ranges (50 to 100 °C) and a special polymer electrolyte
membrane.
How the proton exchange membrane
fuel cell works
Fuel cells essentially transform the chemical energy
released in the reaction between hydrogen and oxygen into electrical energy.
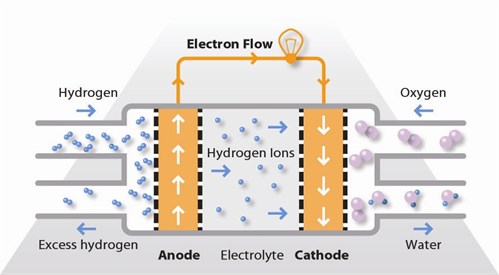
A stream of hydrogen is delivered to the cathode side
of the cell. At the cathode side it is catalytically split into protons and
electrons. An oxidation half—cell reaction takes place reaction (loss of
electrons) at the cathode:
H2
⟶ 2H+ + 2e—
The newly formed protons permeate through the polymer
electrolyte membrane to the anode side. The electrons travel along an external circuit
to the anode side of the cell.
Meanwhile, a stream of oxygen is delivered to the anode
side of the cell.
At the anode side, oxygen molecules react with the
protons permeating through the polymer membrane and the electrons arriving by
the external circuit and combine to form water molecules.
A reduction half-cell reaction or oxygen reduction takes
place at the anode:
½O2 + 2H+ + 2e— ⟶ H2O
The
overall effect is for hydrogen and oxygen to combine to form water:
H2 + ½O2
⟶ H2O
Properties of the polymer membrane
To function, the membrane must conduct hydrogen ions
(protons) but not electrons as this would in effect "short circuit” the
fuel cell.
The membrane must also not allow either gas to pass to
the other side of the cell, a problem known as gas crossover.
Finally, the membrane must be resistant to the
reducing environment at the cathode as well as the harsh oxidative environment
at the anode.
The Direct Methanol Fuel Cell
Direct-methanol fuel cells or DMFCs
are similar to proton exchange fuel cells but methanol is used as the fuel.
Their main advantage is the ease of transport of methanol (over hydrogen gas)
because methanol is an energy-dense yet reasonably stable liquid in most
environmental conditions.
Methanol is a liquid from -97.0 °C to 64.7 °C at
atmospheric pressure. The energy density of methanol is an order of magnitude
greater than even highly compressed hydrogen, and 15 times higher than lithium
ion batteries.
The chemistry of
how the DMFC works
The DMFC relies upon the oxidation of methanol on a Platinum/Ruthenium
catalyst layer to form carbon dioxide.
Water
is consumed at the anode and is produced at the cathode. Protons (H+)
are transported across the proton exchange membrane—often made from polymer—to
the cathode where they react with oxygen to produce water.
Electrons flow
through the external circuit from cathode (—) to anode (+), providing power to
connected devices.
Cathode (—) reaction:
CH3OH + H2O ⟶ 6H+ + 6e— + CO2 Oxidation
Anode (+) reaction:
1½O2 + 6H+ + 6e— ⟶ 3H2O
Reduction
Overall reaction:
CH3OH + 1½O2 ⟶ 2H2O + CO2
You can see that the overall reaction is just the
combustion of methanol in pure oxygen.
Methanol and water are adsorbed on a catalyst usually
made of platinum and ruthenium particles, and lose protons until carbon dioxide
is formed.
As water is consumed at the cathode in the reaction, pure methanol
cannot be used without provision of water via either passive transport such as osmosis
or pumping. The need for water limits the energy density of the fuel.
Cell efficiency is quite low, so they are targeted at
portable applications, where energy and power density are more important than
efficiency.










No comments:
Post a Comment