C4.1
Predicting chemical reactions of the halogens with halide ions
Summary
Models of
how substances react and the different types of chemical reactions that can
occur enable us to predict the likelihood and outcome of a chemical reaction.
The current periodic table was developed based on observations of the
similarities and differences in the properties of elements. The way that the
periodic table is arranged into groups and periods reveals the trends and
patterns in the behavior of the elements. The model of atomic structure
provides an explanation for trends and patterns in the properties of elements.
The arrangement of elements in groups and periods reveals the relationship
between observable properties and how electrons are arranged in the atoms of
each element.
Common
misconceptions
Learners
consider the properties of particles of elements to be the same as
the bulk
properties of that element. They tend to rely on the continuous matter model
rather than the particle model. Learners confuse state changes and dissolving
with chemical changes. Also, since the atmosphere is invisible to the eye and
learners rely on concrete, visible information, this means they therefore often
avoid the role of oxygen in their explanations for open system reactions. Even
if the role of oxygen is appreciated, learners do not realize that solid
products of an oxidation reaction have more mass than the starting solid.
Underlying
knowledge and understanding
Learners
should be familiar with the principles underpinning the Mendeleev periodic
table; the periodic table: periods and groups; metals and non-metals; the
varying physical and chemical properties of different elements; the chemical
properties of metals and non-metals; the chemical properties of metal and
non-metal oxides with respect to acidity and how patterns in reactions can be
predicted with reference to the periodic table.
C4.1a-b
To be able to recall the simple chemical properties of Group 7
To be able to explain how observed simple chemical properties of Group 7
depend on the outer shell of electrons of the atoms and predict properties from
given trends down the groups including the ease of electron gain or loss.
The Halogens form a group of non- metals on the right hand side of the
Periodic Table Group 7 next to the noble gases.
There are three particular chemical reactions that most courses include.
The reaction of halogens with metals
The reaction of halogens with non – metals
The reaction of halogens with halide ions
Then there is the simple chemical test for halide ions.
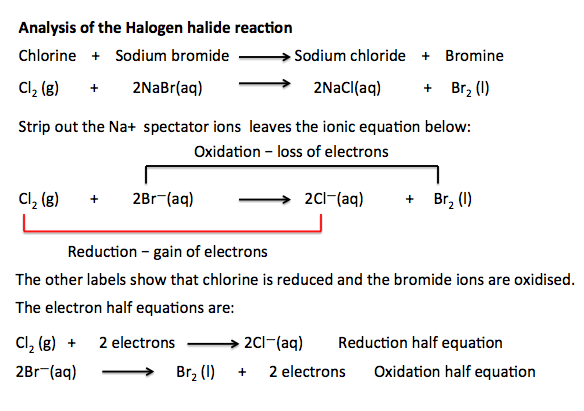
Reaction of the halogens with halide ions
Halogens oxidise halide ions.
The halide ion has to be lower
down Group 7.
The table shows the reactions
between halogens and halides that are possible:
Here is the analysis of the
halogen:halide reaction for the reaction between chlorine and sodium bromide.
The smaller the halogen the
better it is at accepting electrons (it is a better oxidising agent) because it
has fewer electron shells and a higher effective nuclear charge with a stronger
electrostatic pull on any incoming electron that fills the halogen outer shell.
The reactions take place
quickly and the halogen produced can be shown up using an organic solvent such
as hexane of chloroform or cyclohexane.
I’ve grabbed a couple of videos
to illustrate the reactions but you should have done these in a lab yourself
anyway.











No comments:
Post a Comment