New OCR Gateway specification from
September 2016 Higher tier: grades 9 to 4:
In this and subsequent posts I’m simply going to explain and illustrate each learning objective as they come up in the topics in the new GCSE specification.
I’m giving you my notes from each lesson.
You can really get ahead of your class if you follow this blog and all the posts that will appear here about the new GCSEs over the coming months.
This rejigging of the specification is just that: there is nothing really new here it has all been with us for the past half century at least.
That written in italics is for the higher tier paper only.
Hardness
In this and subsequent posts I’m simply going to explain and illustrate each learning objective as they come up in the topics in the new GCSE specification.
I’m giving you my notes from each lesson.
You can really get ahead of your class if you follow this blog and all the posts that will appear here about the new GCSEs over the coming months.
This rejigging of the specification is just that: there is nothing really new here it has all been with us for the past half century at least.
That written in italics is for the higher tier paper only.
C2
Elements, Mixtures and Compounds
C2.3
Properties of materials
C2.3a
recall that carbon can form four covalent bonds
CM2.3i
represent three-dimensional shapes in two dimensions and vice versa when
looking at chemical structures, e.g. allotropes of carbon
Carbon is in Group
4(14) of the Periodic Table.
Group 4 elements have
four electrons in their outer shells.
Four more electrons
would fill the outer-shell of carbon.
Covalent bonds form
between carbon and other elements like hydrogen (H), oxygen (O), carbon (C)
itself, nitrogen (N) and sulfur (S).
These covalent bonds
that form are made up of pairs of electrons shared between carbon and the other
element.
The particles that
form between carbon and these other atoms are called molecules because they are
distinct groups (sometimes very large) of atoms.
Methane (CH4)
is an example of a molecule where carbon and hydrogen atoms share 4 pairs of
electrons.
A Lewis or dot and
cross diagram illustrates this structure:
A line represents each
shared electron pair.
But the molecule is
not this shape in three dimensions.
The illustration below
shows the position of four covalent bonds around the carbon atom in 3D
The illustration above
shows the display formula or simple drawing of methane with the angle between
the fours covalent bonds at 90o
But in 3D the other
two illustrations (ball and stick and space-filling models) show the angle
between the four bonds to be greater at 109½o.
The shape of the
methane molecule is not a flat cross but a triangular based pyramid or
tetrahedron: look at the illustration below:
C2.3b
explain that the vast array of natural and synthetic organic compounds occur
due to the ability of carbon to form families of similar compounds, chains and
rings.
One amazing things
about carbon that is not true of any other element is that carbon atoms bond to
other carbon atoms so well that chains and rings of carbon atoms exist.
The simplest chain of
carbon atoms is C2H6 ethane.
The simplest ring of
carbon atoms is C3H6 cyclopropane.
These molecules have similar
molecular formulae but the atoms are arranged very differently.
Ethane looks like
this:
Cyclopropane looks
like this:
You can see a rotatable 3D
model of cyclopropane here
This illustration
shows some of the simplest chain hydrocarbons.
And this illustration
shows some of the simplest ring carbon molecules
And these are just a
small selection of the millions of chain and ring molecules formed because
carbon can bond strongly to itself and other nonmetals.
C2.3c
explain the properties of diamond, graphite, fullerenes and graphene in terms
of their structures and bonding.
CM2.3i
represent three-dimensional shapes in two dimensions and vice versa when
looking at chemical structures, e.g. allotropes of carbon.
In C2.2d(iii)
there is an explanation of the structure and bonding in diamond
Typical
giant covalent structures are diamond, graphite, nano–tubes and graphene
sheets.
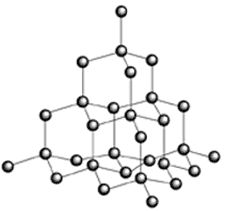
i) Diamond
Four
bonds connect each carbon atom to four others in a tetrahedral arrangement.
These
inter–atomic bonds are very strong.
The
ball and stick model below shows this tetrahedral arrangement of atoms.
The
arrangement is repeated continuously throughout a diamond crystal.
Hardness
The
result is that diamond is the hardest
known naturally occurring material.
Conduction of electricity
It
does not conduct electricity because
all the atom’s electrons and used in forming covalent bonds with other carbon
atoms.
Melting and boiling points
Its
melting and boiling points are
incredibly high because to melt or boil diamond each individual strong bond
has to be broken and that would take a vast amount of energy.
ii) Graphite
Three
bonds connect each carbon atom to three others in a sheet of atoms arranged in
hexagons.
These
inter–atomic bonds are very strong.
But
the sheets bond weakly to each other so that the sheets can easily slide over
each other.
As
with diamond the arrangement is repeated through a crystal of graphite.
The
giant structure is evident in this picture below:
The
weak bonds between the layers of carbon atoms allow these layers to slide over
each other making graphite soft and
useful in pencil “lead” and in oils as a lubricant.
Conduction of electricity
As
only three bonds hold each carbon atom in place in the layers there is one
electrons per carbon atom free (or delocalized) that allows for the conduction of electricity.
Melting and boiling points
But
its melting and boiling points are very
high since again, as in the case of diamond, every strong bond in each
layer has to be broken for the material to melt or boil and this would take an
incredibly high amount of energy.
iii) Nano tubes and fullerenes
Nano-tubes
are tubes of rolled up graphite sheets.
Each
carbon atom is bonded to three others using three strong bonds.
The
carbon atoms are arranged in hexagons.
Fullerenes
are spheres of carbon atoms composed of hexagons and pentagons.
These
molecules of carbon C60, C70 etc. have strong bonds
within the molecule but weak bonds between the molecules.
The
melting and boiling points of fullerenes are low because not much energy is
needed to separate one molecule from another due to these weak inter molecular
bonds.
For
this reason too fullerenes are soluble in organic solvents like petrol. C60 forms a red solution in petrol
(octane).
iv) Graphene
Graphene
is a single sheet of graphite.
A
one atom thick layer.
The
carbon atoms are arranged in hexagons and each has three bonds connecting it to
three others.
So we would expect
grapheme to be very strong as the bonds holding the carbon atoms in the sheet
are very strong.
We’d also expect the
sheet to conduct electricity since only three bonds hold each atom in place
leaving a free electron to form the electric current.
There are many sites
on the web where you can pick up further detail about the allotropes of carbon. Here are just two links you could follow.






















No comments:
Post a Comment