In a previous post I asked this question:
How come the angles between the bonds in methane are all 109.5°?
When the molecule of methane is examine closely it has a particular shape.
We can see that shape on this picture of a model of the molecule:
The shape is called a tetrahedron or a triangular based pyramid.
How come the angles between the bonds in methane are all 109.5°?
When the molecule of methane is examine closely it has a particular shape.
We can see that shape on this picture of a model of the molecule:
Imagine the carbon atom is in the centre and the hydrogen atoms are at the points of the tetrahedron:
Now how come this configuration fits the molecule?
Remember each of the bonds is formed from a pair of electrons that are negatively charged.
Like all negative charges these electrons will repel each other to the point where they cannot repel any further.
Therefore, the molecule adopts the tetrahedral shape that results from the maximum repulsion of the bond pairs of electrons.
In the case of methane the hydrogens end up at the points of a tetrahedron.
The bond angle can be calculated from geometrical theory to be 109.5°.
But what if the molecule contains both bonds and non bonding electrons, what happens then?
Let's look at the example of ammonia NH3
What is the shape of the ammonia molecule and what is its bond angle?
Here is the dot and cross diagram of ammonia:
You can see there are three bond pairs but also one pair of electrons is not bonded to hydrogen.
This is a non bonding pair of electrons.
Non-bonding pairs of electrons exist closer to the nucleus than bonding pairs of electrons or you can say they take up more "bonding space".
Therefore, non-bonding pairs of electrons exert a greater repulsive force on the bond pairs than the bond pairs do on each other.
How does that affect the shape of the ammonia molecule and its bond angle?
As you can see from the diagram below the 4 pairs of electrons are like a tetrahedron but the non-bonding pair push the bond pairs closer to each other.
So the angle between the N-H bonds is not 109.5° but they exist a little closer to each other at 107.3° apart.
The resultant shape is called pyramidal or trigonal pyramidal.
Non bonding pairs of electrons repel bond pairs more than bond pairs repel bond pairs.
And if a molecule has two bond pairs and two non-bonding pairs then the angle between the bond pairs will be reduced even further.
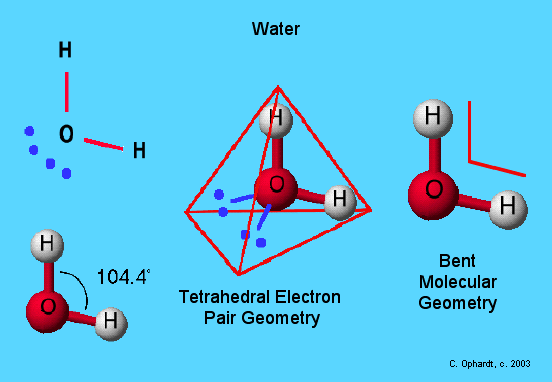
Here is the example of water H2O:
As you can see the effect of the two non-bonding pairs is to reduce the H-O-H bond angle even further than in ammonia to 104.4°.
Water is described as a bent or angular molecule.
The theory that gives rise to these shapes is called Valence Shell Electron Pairs Repulsion theory or VSEPR for short.
Here is a formal statement of the VSEPR theory:
The VSEPR theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence or outer shell of that atom. Non- bonding electron pairs exert greater repulsion than bond pairs and double bonds (2 electron pairs) exert greater repulsion than do single bonds.
So how can we work out the shape of a simple molecule or ion?
1) Work out the number of electrons in the outer shell of its central atom.
2) Work out the number of atoms that each add one electron to fill the outer shell
3) Add together answers to 1 and 2 then divide by two to find the number of bond pairs
4) Use the table below to read off the shape of the simple molecule
Electron pairs Molecule geometry Example
2 linear BCl2
3 trigonal
planar BF3
4 tetrahedral CH4
5 trigonal
bipyramidal PF5
6 octahedral SF6
The theory has to be modified to account for non-bonding pairs and multiple bonds.
a) Non bonding electrons exert a greater force on bond pairs and have the effect of distorting the basic geometries of molecules.
Calculating the number of electrons in the outer shell of the central atom that are not bonded will usually determine the effect of these non bonding electrons on the molecule shape.
e.g. H2S in which there are two non bonding pairs and two bonding pairs giving rise to a shape similar to that of water.
Why the angle is smaller than that of water I'm going to leave to an upcoming blog on bond hybridisation theory.
Here you can use water as an analogue to that of hydrogen sulphide.
b) Multiple bonds have greater electron density and also exert a greater force on single bond pairs.
Let's look at the bond angles around one of the carbon atoms in ethene, C2H4.
There are 4 electrons around the carbon atom
There are two electrons from the two hydrogen atoms attached to this carbon
There are two single bonds then and a double bond
The molecule has 3 bonds attached to each carbon so it is basically trigonal in shape
But the double bond has a greater density of electrical charge so pushes the two bond pairs together slightly reducing the 120° angle. Like this:
There are 4 electrons around the carbon atom
There are two electrons from the two hydrogen atoms attached to this carbon
There are two single bonds then and a double bond
The molecule has 3 bonds attached to each carbon so it is basically trigonal in shape
But the double bond has a greater density of electrical charge so pushes the two bond pairs together slightly reducing the 120° angle. Like this:
You can calculate the H-C-H bond angle to be 117.4° slightly less than the standard trigonal planar angle of 120°.
New Pages on the "Mole" and "Using the Mole" in chemical calculations are here
















No comments:
Post a Comment