Chemical Bonding: covalent bonds in alkanes like methane.
Looking at some of those formulae we've used in the previous oil fractionation posts should leave us puzzled.
What do the lines joining the element symbols stand for?
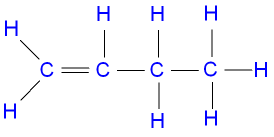
Here's a displayed formula for Butene that shows all the atoms and all the bonds in the molecule:
So what do the lines stand for?
Our explanation goes right back to the Periodic Table.
Let's look at a Periodic Table of just the first 20 elements.
Here we have names and symbols of the first twenty elements each with a couple of numbers:
It's the numbers we need to be looking at first especially the top one called the atomic number (symbol Z)
This is the number of protons (positively charged) in each atom's nucleus.
And since all atoms are electrically neutral it is also the number of each atom's electrons (negatively charged).
Your bog standard helium atom (He), you guessed it, has two protons and two electrons.
If you want to know about the other number (called Relative Atomic Mass or RAM for short) you'll need to go here.
Why is the number of electrons important to us?
Because that's what allows atoms to combine or stick or bond to each other.
There are several different ways in which the bonds can form but we are just thinking in this blog about the way atoms like hydrogen and carbon bond together.
And they bond like this:
The atoms in the Periodic Table hold their electrons in specific groups called shells or energy levels.
These shells or energy levels obey fixed rules.
The first shell can only hold two electrons, the second shell eight electrons and the third shell 18 electrons.
Let's see how this works out for the first twenty elements:
Here you can see the numbers in red are the numbers of electrons in each shell of that atom.
For example carbon 2)4 has six electrons, 2 in the first shell and 4 in the outer shell.
No prizes for guessing which element is missing form this illustration (Thanks BBC Bitesize). Yes, you guessed it, Hydrogen.
Hydrogen has just one electron orbiting the nucleus.
So how is it that carbon and hydrogen can bond together to make methane?
If the outer shell electrons are shared between hydrogen and carbon, both elements gain full outer shells.
That's eight for carbon and two for hydrogen.
A full outer shell is considerably more energetically stable because the shells are at lower energies so that's why they form.
We construct what we call dot and cross diagrams to show this new situation, though the Americans call the diagrams Lewis Diagrams after GN Lewis who first used them.
Here's the one for methane:
So now you should be able to see that the line in the displayed formula for methane stands for a pair of electrons shared between hydrogen and carbon.
You can see them shown as red and green dots in the diagram on the left.
Pairs of shared electrons like this are called covalent bonds.
Sometimes you'll see them as dot and cross diagrams where the electron from one atom is shown by a cross and the electron from the other atom is shown by a dot.
Like here:
You could try to construct Dot and Cross diagrams for other simple molecules, why not try water (H2O), ammonia (NH3) and chlorine (Cl2)?
Students working on more advanced courses could also build Lewis diagrams for ethane (C2H6), ethene C2H4) with its double carbon carbon bond, and chloromethane (CH3Cl).
In the next blog, Chemical Bonding (2), I will talk about the shapes of molecules composed of covalent bonds, asking how it is that the angle between the covalent bonds in methane is exactly 109.5°?
Pages on the "Mole" and "Using the Mole" in chemical calculations are here
Looking at some of those formulae we've used in the previous oil fractionation posts should leave us puzzled.
What do the lines joining the element symbols stand for?
Here's a displayed formula for Butene that shows all the atoms and all the bonds in the molecule:
So what do the lines stand for?
Our explanation goes right back to the Periodic Table.
Let's look at a Periodic Table of just the first 20 elements.
It's the numbers we need to be looking at first especially the top one called the atomic number (symbol Z)
This is the number of protons (positively charged) in each atom's nucleus.
And since all atoms are electrically neutral it is also the number of each atom's electrons (negatively charged).
Your bog standard helium atom (He), you guessed it, has two protons and two electrons.
If you want to know about the other number (called Relative Atomic Mass or RAM for short) you'll need to go here.
Why is the number of electrons important to us?
Because that's what allows atoms to combine or stick or bond to each other.
There are several different ways in which the bonds can form but we are just thinking in this blog about the way atoms like hydrogen and carbon bond together.
And they bond like this:
The atoms in the Periodic Table hold their electrons in specific groups called shells or energy levels.
These shells or energy levels obey fixed rules.
The first shell can only hold two electrons, the second shell eight electrons and the third shell 18 electrons.
Let's see how this works out for the first twenty elements:
Here you can see the numbers in red are the numbers of electrons in each shell of that atom.
For example carbon 2)4 has six electrons, 2 in the first shell and 4 in the outer shell.
No prizes for guessing which element is missing form this illustration (Thanks BBC Bitesize). Yes, you guessed it, Hydrogen.
Hydrogen has just one electron orbiting the nucleus.
 |
| methane formulas and models |
If the outer shell electrons are shared between hydrogen and carbon, both elements gain full outer shells.
That's eight for carbon and two for hydrogen.
A full outer shell is considerably more energetically stable because the shells are at lower energies so that's why they form.
We construct what we call dot and cross diagrams to show this new situation, though the Americans call the diagrams Lewis Diagrams after GN Lewis who first used them.
Here's the one for methane:
So now you should be able to see that the line in the displayed formula for methane stands for a pair of electrons shared between hydrogen and carbon.
You can see them shown as red and green dots in the diagram on the left.
Pairs of shared electrons like this are called covalent bonds.
Sometimes you'll see them as dot and cross diagrams where the electron from one atom is shown by a cross and the electron from the other atom is shown by a dot.
Like here:
You could try to construct Dot and Cross diagrams for other simple molecules, why not try water (H2O), ammonia (NH3) and chlorine (Cl2)?
Students working on more advanced courses could also build Lewis diagrams for ethane (C2H6), ethene C2H4) with its double carbon carbon bond, and chloromethane (CH3Cl).
In the next blog, Chemical Bonding (2), I will talk about the shapes of molecules composed of covalent bonds, asking how it is that the angle between the covalent bonds in methane is exactly 109.5°?
Pages on the "Mole" and "Using the Mole" in chemical calculations are here














No comments:
Post a Comment