GCSE OCR Gateway Chemistry
C6.1p Corrosion
C6.1p To be able
to describe the process of corrosion and the conditions that cause
corrosion in iron and other
metals.
Corrosion in iron and other metals
Causes and conditions of the corrosion of
iron
Iron easily
corrodes to form brown rust a flaky substance that peels off the surface of the
exposed iron.
It is
possible to show that rust is produced from the action of both water and oxygen
on the surface of the iron.
Chemically
the reaction is an oxidation type reaction in which hydrated iron(III) oxide is
formed as in the typical equation below:
4Fe + xH2O
+ 3O2 ⟶ 2Fe2O3.xH2O
This
reaction can be confirmed using the following experiment in which simple test
tube reactions are set up with either air or water removed. In both cases, there is a reduction or a
complete absence of the formation of rust.
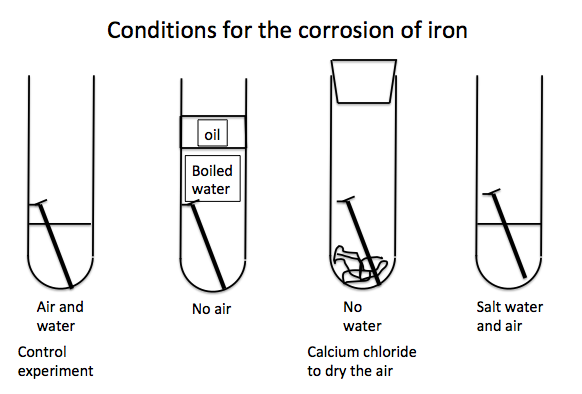
The first
test tube is the control experiment in which there is an iron nail immersed in
water and in contact with air. The iron
nail rusts in this tube after a couple of days.
The second
tube contains an identical iron nail in water which has been boiled for a
significant length of time in order to remove all dissolved oxygen. This set up is then sealed with a layer of
oil to prevent air re-dissolving in the water.
No corrosion is observed after several days.
The third
tube contains an identical iron in absolutely dry air. Calcium chloride (CaCl2) in the
tube removes all water from the air in the tube. After several days the iron nail has not
corroded.
The fourth
tube contains the same nail this time immersed in salt water and in contact
with air.
If salt
water is used instead of water in the control experiment then we observe an
acceleration of rust formation. We see this in the results of the experiment in the photo below:
These
results to this simple experiment suggest that water and air are required for
iron to corrode.
Corrosion in some other metals
Other metals
do corrode but not as severely or as rapidly as iron
Copper
Copper we
know and see turns green on exposure to air and rainwater. Copper looks very attractive when first constructed as we see below:
Aluminium













No comments:
Post a Comment