New OCR Gateway specification from
September 2016 Higher tier: grades 9 to 4:
In this and subsequent posts I’m simply going to explain and illustrate each learning objective as they come up in the topics in the new GCSE specification.
I’m giving you my notes from each lesson.
You can really get ahead of your class if you follow this blog and all the posts that will appear here about the new GCSEs over the coming months.
This rejigging of the specification is just that: there is nothing really new here it has all been with us for the past half century at least.
That written in italics is for the higher tier paper only.
In this and subsequent posts I’m simply going to explain and illustrate each learning objective as they come up in the topics in the new GCSE specification.
I’m giving you my notes from each lesson.
You can really get ahead of your class if you follow this blog and all the posts that will appear here about the new GCSEs over the coming months.
This rejigging of the specification is just that: there is nothing really new here it has all been with us for the past half century at least.
That written in italics is for the higher tier paper only.
C2
Elements, Mixtures and Compounds
C2.3
Properties of materials
C2.3d
use ideas about energy transfers and the relative strength of chemical bonds
and intermolecular forces to explain the different temperatures at which
changes of state occur.
C2.3f
explain how the bulk properties of materials (ionic compounds; simple molecules;
giant covalent structures; polymers and metals) are related to the different
types of bonds they contain, their bond strengths in relation to intermolecular
forces and the ways in which their bonds are arranged.
More on this topic can be found here
Let’s look at five typical
substances:
An ionic substance:
sodium chloride mp 808oC, bp 1465oC
A simple molecular
substance: water mp 0oC, bp 100oC
A giant covalent
molecular substance: diamond which sublimes above 5000oC
A polymer: polythene
mp 115–135oC, bp 388–408oC
A metal: copper mp
1085oC, bp 2562oC
Ionic
substances: sodium chloride NaCl
When ionic substances
melt the positive and negative ions separate.
The regular
arrangement of ions breaks down but the ions still stay close to each other,
probably in random clusters.
Heating sodium
chloride to 808oC gives the substance enough energy to break the
electrostatic attraction of the ions for each other.
If the temperature is
raised to 1465oC then the ions have gained sufficient energy to fly
apart from each other, completely overcoming the electrostatic attraction of
positive ion for negative ion, and enter the gaseous state.
The lattice energy represents
the strength of all the bonds between all the ions in an ionic substance.
The Lattice energy of
sodium chloride is +787kJ/mol. This
means that the energy needed to take all the ions apart in a mole of sodium
chloride is 787kJ.
If the ions have twice
the charge (+2 and –2) but the substance has same structure then the lattice
energy.
Magnesium oxide (MgO) has
the same structure as sodium chloride but each ion has twice the charge so the
ionic bond between all the ions is much stronger and its lattice energy is much
higher: 3800kJ/mol.
Magnesium oxide then
has higher melting and boiling points than sodium chloride.
Magnesium oxide: mp
2852oC, bp 3600oC.
This kind of behavior where
increased ion charge leads to increases in melting and boiling point is true
for those ionic substances that do not decompose on heating such as chlorides
and oxides.
But it is not true for
those ionic substances that do decompose on heating carbonates or nitrates or
sulphates .
Ionic substances are
usually hard because the electrostatic forces between the ions are strong.
Ionic substances
usually conduct electricity when molten because the ions have become free to
move around and can be attracted to positive and negative electrodes.
Some ionic substances
are soluble in water because the water molecules can bond electrostatically to
their ions.
In other ionic
substances the forces between the ions are too strong (as in magnesium oxide)
to allow the ions to separate in the presence of water so these ionic
substances are insoluble in water.
Simple
Molecular substances: water
Simple molecular
substances contain two types of bond.
There are very strong
covalent bonds between the atoms in the molecule i.e. in water there are strong
bonds between the oxygen atom and the two hydrogen atoms.
Then there are much
weaker bonds between molecules in the solid state.
The intermolecular
bonds are sometimes called van der Waals forces or London forces.
When ice is heated the
energy given to the substance first breaks the intermolecular bonds.
We can tell these
bonds are weak because the melting point is very low at 0oC.
If the substance is
heated further it will boil at 100oC because the intermolecular bonds between the molecules in water
are weak.
Many simple molecular
substances behave just like water substances such as ethanol, methane,
propanone or carbon dioxide.
All have strong
covalent bonds between atoms and weak bonds between molecules.
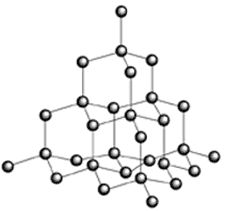
Giant
Molecular substances: Diamond
Giant molecular
substances have a structure made up of all strong covalent bonds in a network
formation.
Since the covalent
bonds between the atoms are all the same strength and very strong it takes a
considerable amount of energy to break them.
So if diamond is to
melt or boil all the strong covalent bonds between all the carbon atoms in a
diamond crystal are going to have to break.
What in fact happens
when diamond is heated is that its atoms separate from the structure at about 5000oC
and the diamond becomes a gas.
Moving from solid to
gas without passing through the liquid state is called sublimation.
You can see good animations of the
diamond structure here.
Diamond is hard
(hardest natural substance) because of the very strong covalent bonds between
the carbon atoms and the arrangement where each atom is held in place by 4 of
these strong covalent bonds. The
arrangement of atoms–a tetrahedral arrangement–also lends strength to the
substance.
A
polymer: polythene mp 115–135oC, bp 388–408oC
Polymers are long chain
like structures of atoms usually carbon based.
A polymer has a
pattern of repeating atoms–a repeat unit.
Repeat units are based
on the molecule used to form the polymer the monomer.
Polythene has ethene C2H4
for its monomer.
Join the repeat units
at the stars and you build the polymer structure that in part could look like
this:
There are strong
covalent bonds between the atoms of the polymer but thousands of weak bonds
between the polymer chains.
The polymer chains are
never identical, some are longer some are shorter than others, so the number of
weak bonds between the chains varies and that means the melting and boiling
points of different chains will vary.
The amount of
crystallinity in the polymer also affects the melting and boiling point.
More crystallinity
probably raises the melting point and boiling point because the polymer chains
will be more ordered and closer together so the bonds between them will be
stronger.
Overall these two
factors mean that the melting and boiling points of polymers are in a range of
values and not precise.
A
metal: copper mp 1085oC, bp 2562oC
Metal melting and
boiling points are very precise unlike those of polymers.
Bonding in metals
depends on the number of outer shell electrons.
When bonds form
between atoms the atoms’ outer shells overlap to form one complete “sea of
electrons” or a “cloud of electrons” or sometimes called delocalised electrons.
So the electron cloud
bonds together the remaining particles that are positively charged ions.
The positive ions and
sea of electrons are in the form of a 3D lattice or network and are like a
climbing frame in the local park.
The sea of electrons
allows the metal particles to move around each other whilst remaining bonded.
As result we can
easily bend a piece of metal and stamp it and reform it into a new shape.
C2.3e
use data to predict states of substances under given conditions.
Substance
|
Mp (oC)
|
Bp (oC)
|
State at room
temperature
|
State at 100oC
|
State at 1000oC
|
Sodium chloride
|
808
|
1465
|
Solid
|
Solid
|
liquid
|
water
|
0
|
100
|
liquid
|
gas
|
gas
|
diamond
|
5000+
|
5000+
|
Solid
|
Solid
|
Solid
|
Magnesium oxide
|
2852
|
3600
|
What are the states of
magnesium oxide at the three temperatures?