I
wonder if we know just how common amides
are?
Let
me ask you: do you like cooked chicken, roast beef or lamb, grilled fish or are
you having a barbeque somewhere in the world as I write this?
Then
you are eating amides in the form of protein.
The
human body proteins are essentially polyamides like keratin in hair, collagen
in muscle, haemoglobin in blood.
You
and I could be described as bundles of protein, bundles of amides.
So
a study of amides bears paying some attention, do you not think?
I’m
going to describe essential structure and formation of amides and discuss some
more relevant and contentious examples.
Structure and
Formation
An
amide is a carboxylic acid derivative
formed from the reaction between an acid chloride and ammonia.
This
is a nucleophilic addition-elimination
reaction.
Here
is the mechanism:
The amide group is –CONH2
The
structure can be written:
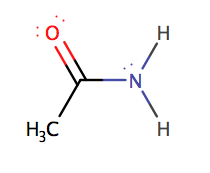
Here
is ethanamide:
Amides also form condensation polymers.
The following polymers are polyamides:
The following polymers are polyamides:
Nylon,
Kevlar, all proteins, but don’t confuse nylon with the Nylon publication:
Nylon
Synthesis
of a typical nylon:
Nylon
6:10 is called 6:10 because the two monomers have 6 and 10 carbon atoms
respectively.
One
monomer is decandioyl dichloride,
the other 1,6–diaminohexane.
Notice
the peptide bond (–CONH–) in the polymer repeat unit.
This
example reaction forms the chemistry of what is sometimes called the Nylon Rope Trick that you can see here on You Tube.
You
need to listen carefully to the spiel because the professor makes a couple of mistakes.
The
diamine is in the lower aqueous
solution put in the flask first and the solution he adds to the flask is the acid dichloride dissolved in hexane.
He
has it the other way round, wrongly.
If
you want to repeat this experiment for yourself you can find it here
on the RSC site
Nylon
has great strength.
The
polymer chains can hydrogen bond to
each effectively through the peptide linkages.
The
long alkyl groups also bond quite well through van der Waals forces.
There
is a fine myth about Nylon as to how
it got its name.
You
can read about that myth here
on the Timeless Myths website.
I
won’t spoil the party for you.
There
are no spoilers here!!
Kevlar
Let’s
look now at Kevlar
Here
is the formation of Kevlar:
Kevlar
is a constituent of bulletproof vests.
You
can see that the polymer chains have many phenyl rings.
These
rings bond the chains strongly together through the use of many van der Waals forces.
The
peptide linkages also bond strongly through Hydrogen bonding like this:
The
result is a very tough impenetrable material.
Can
you work out what the monomers of Kevlar would have to be?
Kevlar uses
Proteins
All
proteins are polyamides but their monomers are not diamines and acid
dichlorides.
Amino acids are the protein
monomers.
And
interestingly there are some amino acids that are essential amino acids that we must take in our diet because they
are not made in our bodies.
They
are histidine, isoleucione, leucine, lysine, methionine, phenylalanine,
threonine, tryptophan and valine.
You
can read more about essential and non–essential aminoacids here
Here’s
how amino acids polymerise:
The
convention is to draw them starting with the amino group on the left.
Note
also the peptide bond that unites both amino acids.
The
condensation reaction eliminates a small molecule of water (not shown) from
between the two amino acids.
A
string of amino acid residues linked to one another using peptide bonds is the primary structure of a protein.
Amides as drugs
N-phenylethanamide
is an anti–coagulant.
It
prevents the clotting and coagulation of red blood cells.
Its
formation involves reaction between phenylamine and ethanoyl chloride or
ethanoic anhydride.
Substituted
versions of this molecule have been used in the treatment of arthritis.
Aspartame
Aspartame
is an artificial sweetener or sucrose substitute.
It
is a dipeptide and here is its structure:
This internet clip shows the peptide bond highlighted in a green box.
This
clip shows that the molecule is a dipeptide formed from two amino acids
aspartic acid and phenylalanine.
It
also shows that it is the methyl ester of phenylalanine.
So
far all rather innocuous and conventional.
OK let's use it as an artificial sugar substitute. That's what Splenda is formed from:
But……
You see this on the internet and this:
It's
just that “the lady doth protest too much methinks” to quote Shakespeare’s
Queen Gertrude in Hamlet.
Are
we really to believe that all these conditions are solely attributable to
aspartame.
What
about the dangers of sucrose?
But of course, in today’s suspicious world everyone is thought to have a hidden agenda and cannot therefore be trusted.
And
what is there that we eat that does not cause
us issues?

























No comments:
Post a Comment