NMR
spectroscopy is one of the most versatile and valuable non-invasive
investigative techniques chemists have these days to identify and put together
the structures of molecules.
How does it work?
This
is not an easy explanation for school and college students to get a hold of.
I’m
just going to discuss 1H proton nmr spectroscopy but if you delve
deeper into this topic you will come across equally valuable 13C and
31P nmr as well.
You'll notice that these species all have an odd number of nucleons.
So if the proton is Deuterium with two nucleons this does not register in nmr.
You'll notice that these species all have an odd number of nucleons.
So if the proton is Deuterium with two nucleons this does not register in nmr.
Background NMR
theory
Proton
nmr tells us about the chemical environment of 1H hydrogen nuclei in
molecules.
1H hydrogen nuclei
spin about an axis.
They
have spin quantum number ½
As
they spin, the positive charge moves and creates its own very small magnetic
field.
Put
this spinning 1H hydrogen nucleus in an external magnetic field and
it will either spin so as to line itself up with this external magnetic field or
it will spin against the field.
What
happens is like what happens when you put a compass needle in the Earth’s
magnetic field and the needle lines up with it pointing north.
Now
back to the proton, if the nucleus is lined up with the external field it has quantum number + ½ .
But
if the spinning nucleus is aligned against
the external field it has quantum number – ½ .
In
fact, most nuclei line up with the field
and far fewer line up against it.
What
this means is that there is an energy gap, ∆E, between the two states of the
spinning nucleus.
Radiation of the right frequency (low frequency radio waves) will match ∆E and lift some nuclei from spin state + ½ to spin state – ½: this is said to ‘flip’ the nuclei.
Energy
is released (it's called relaxation) to the surroundings or other nuclei as the nuclei return to their
original ground state.
This energy registers as an nmr pulse in the nmr spectrum.
What happens in an
actual molecule to the 1H protons if placed in an external
magnetic field?
In
an external magnetic field, the electrons around each 1H nucleus in the molecule
move and flow to produce a local, very small magnetic field that opposes the
applied field.
This
means that all the 1H nuclei in the molecule feel
a magnetic field slightly smaller than the one applied to the molecule.
Each
1H nucleus is then in a different
magnetic environment according to the number and position of the electrons
around it.
These
very slight differences in magnetic field show up in the nmr spectrum as the
nuclei flip and relax.
These
differences are very small for 1H nuclei — in parts per million
(ppm) of magnetic field density B.
However,
it is possible to measure these so-called chemical
shifts ∂.
The
chemical shift ∂ value gives a sense of how much the electrons around the 1H
proton have “shielded” it from the
full effect of the applied external magnetic field.
The more shielding, the larger the chemical shift ∂ value and the lower ∆E.
The chemical shift ∂ values need a reference point.
The chemical shift ∂ values need a reference point.
The
magnetic signal from Tetra Methyl Silane
TMS (CH3)4Si is used as a reference as its protons
are hardly shielded from the effect of the external magnetic field.
tetramethylsilane
All
other magnetic signals of chemical shift ∂ are the result of the electrons in
the molecule shielding that particular 1H proton more or less.
You
can see here the effect of the π system of electrons above and below the carbon ring.
Table
of chemical shifts are available on the internet.
Equally,
if there is a very electronegative atom
in a molecule its electrons will also shield any 1H protons and
these protons will resonate at higher chemical shift ∂ values.
C1H—R
C1H—Cl
∂ 1.5 ∂ 4.2
Spin–spin coupling
Another
thing can happen if there are hydrogen atoms on different carbon atoms next to
each other in the molecule.
The
signal from one 1H proton will split
the signal from the other proton and vice versa.
Usually,
the effect just happens over a distance of one C–C bond.
The
effect is called spin–spin coupling.
To
predict how the signal from the one proton will split the signal from the other
you have to count the number of protons
on the adjacent carbon atom.
In
simple cases a single signal will split (n+1) times where n is the number of 1H
hydrogen atoms on the adjacent carbon atom.
For
example: Ethanol CH3CH2OH
The
signal from the hydrogen atoms on the –CH2 – group will split the
signal from the CH3 – group because it will interact with the
magnetic field produced by the CH3 – group.
If
we use the (n+1) rule then n for the CH2 group is 2+1 =3 so that the
signal from the CH3 group will be split into a triplet.
Conversely,
the –CH3– group (n+1 or 3+1=4) will split the signal from the –CH2
– group into a quartet.
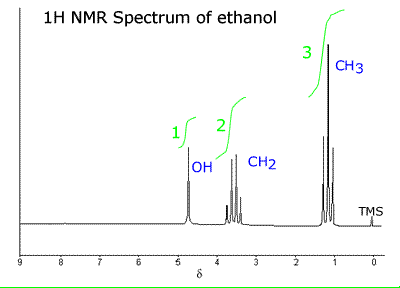
Here
then is the nmr spectrum of ethanol:
You
can see several things here.
First,
the –O–H group is a singlet because the oxygen decouples the hydrogen from the
others in the molecule so there is no spin–spin coupling effect/no peak
splitting.
Second,
the chemical shift ∂ for the –O–H hydrogen is shifted to 4.7 because of the
electrons on the electronegative oxygen atom.
Third,
the CH2 is a quartet as we predicted.
Fourth,
the CH3 is a triplet as we predicted.
Fifth,
the peak areas (given in green) show us that they are in the ratio of the
number of hydrogen atoms on each carbon.
Sixth,
the intensity of the peaks take up the pattern of Pascal’s triangle.
Seventh, if the molecule were CH3CH2OD there would be no singlet because of the deuterium atom.
Seventh, if the molecule were CH3CH2OD there would be no singlet because of the deuterium atom.
At
school and college level probably the most complex molecule you will have to
deal with is something like butanone or ethyl ethanoate.
Here
are examples of both nmr spectra below:
Butanone
Ethyl acetate
Some important chemical shifts:
OH in alcohols ∂ between 2 and 4
OH in carboxylic acids ∂ between 10 and 12
Alkane CH between ∂ 0.25 and 1.75 but note: CH > CH2 > CH3
O–CH in alcohols ∂ between 3 and 4
Halogen –CH in halogenoalkanes ∂ between 2.2 and 4.2
Beware symmetrical molecules such as CH2OH CH2OH which has only two peaks and (CH3)4C which has just one peak (like TMS)
You’ll also find many examples of spectra if you click here.
OH in alcohols ∂ between 2 and 4
OH in carboxylic acids ∂ between 10 and 12
Alkane CH between ∂ 0.25 and 1.75 but note: CH > CH2 > CH3
O–CH in alcohols ∂ between 3 and 4
Halogen –CH in halogenoalkanes ∂ between 2.2 and 4.2
Beware symmetrical molecules such as CH2OH CH2OH which has only two peaks and (CH3)4C which has just one peak (like TMS)
You’ll also find many examples of spectra if you click here.
Some important chemical shifts















No comments:
Post a Comment