Ionic
Bonding (1) Evidence for the existence of Ions
In this blog, I want to show you the evidence that exists to suggest that ions are real
particles.
You might
have carried out an experiment like this in your laboratory:—
•Take a
microscope slide and cover with wet filter paper.
•At the mid
point place a crystal of potassium manganate(VII).
•Connect each
of the short sides of the slide to a 20v dc power source and wait.
You can see
a similar experiment done
here
You can find
instructions and further discussion of the manganate(VII) experiment here. Or
here and a good example of the experiment from the Royal Society of
Chemistry here.
Here is a
picture (its fairly stylised) of the kind of result you might expect:
If the
purple colour moves towards only one of the electrodes (the positive electrode) what does that tell us about the particles that make up the purple colour?
Yes, that's
right, they could only be negatively charged.
And we are
pretty sure potassium particles are colourless given how many colourless salts
there are of potassium.
Here is
another experiment you can do fairly easily though it takes time to develop and
see the results:—
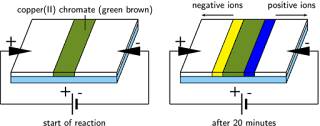
•Place a
paste of copper(II)chromate in the bottom of a U tube.
•Top up each
arm of the U tube with conducting liquid and insert charcoal electrodes
•Set up a 20v
dc potential difference across the electrodes and watch.
After 20mins
or so you might see movement of colour blue to the negative terminal and yellow
to the positive terminal.
These
diagrams illustrate the results of this demonstration:
Here’s
further evidence, as if we needed it, to show that copper ions (blue) are
positively charged species and that chromate(VI) ions (yellow coloured) are
negatively charged species.
One final
piece of evidence to suggest that charges atoms called ions do really exist is
the phenomenon of the electrolysis of molten salts like lead bromide, PbBr2
You can find
details of the experiment and how to carry it out
here
And you can
see the experiment itself
on video here
Here is a
diagram of what we think is happening in the electrolysis of lead bromide:
What I like
about this picture is that it is not too fussy and crowded with detail.
Lead is
produced at the negative cathode suggesting that lead ions carry a positive
charge.
Bromine gas
(highly toxic) is evolved at the anode suggesting bromide ions carry a negative
charge,
The
magnified diagram on the left shows that we think bromide ions lose electrons
to the anode and the resulting atoms pair up to form bromine molecules:
2Br— =
Br2(g) + 2e–
On the
right, the magnified diagram shows positive lead ions each accepting two
electrons.
Pb2+ +
2e– = Pb(l)
The evidence
for the existence of ions is pretty convincing.
The word ION
(he derived it from the Greek for “going”) was first conceived by Michael
Faraday in 1834 in his early experiments with electrolysis in the 19th
century.
Cations (positive
ions) Faraday so named because they go to the negative cathode and anions
(negatively charged) because they go to the positive anode.
This is a
brief introduction into the existence of ions but there is much more
to say about how they enable the formation of compounds and all that will be
found in forthcoming blogs.
Best now go
and do an ion experiment and see them move for yourself.
Happy experimenting!!












No comments:
Post a Comment