Specification extracts:
OCR
Phenols
6.1.1.
- (i) the electrophilic substitution reactions of phenol:
- (i) with bromine to form 2,4,6-tribromophenol
- (ii) with dilute nitric acid to form a mixture of 2-nitrophenol and 4-nitrophenol
- Note that nitration with phenol does not require concentrated HNO3 or the presence of a concentrated H2SO4 catalyst.
- (j) the relative ease of electrophilic substitution of phenol compared with benzene, in terms of electron pair donation to the π-system from an oxygen p-orbital in phenol (see also 4.1.3 a)
Explanation is only in terms of susceptibility of ring to 'attack' and not in terms of stability of intermediate. - (k) the 2- and 4-directing effect of electron- donating groups (OH, NH2) and the 3-directing effect of electron-withdrawing groups (NO2) in electrophilic substitution of aromatic compounds
Learners will not be expected to know further electron-donating or electron-withdrawing groups; relevant additional data will be supplied in examinations. - (l) the prediction of substitution products of aromatic compounds by directing effects and the importance to organic synthesis
Edexcel
6. understand the reaction of phenol with bromine water
7. understand reasons for the relative ease of bromination of phenol, compared to benzene
Aromatic Chemistry (5) Phenol: electrophilic substitution reactions
In this fifth post on aromatic chemistry, I’m looking at the electrophilic substitution reactions of phenol with bromine water and nitric acid.
I’m going to discuss the fact that phenol is more easily substituted than benzene and explain this difference illustrating it by looking at the reaction with bromine water and with nitric acid solution.
Phenol with bromine water
Phenol is an aromatic alcohol but it behaves very differently from benzene in electrophilic substitution reactions.
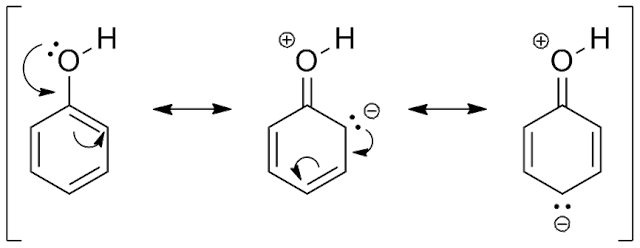
The structure of phenol (C6H5OH) means that the OH group attached to the benzene ring can add extra electrons to the ring π system and as result enhance the electron density at certain of the carbon atoms in the ring. (see below).
The additional electron pair comes from a p orbital in the oxygen of the OH group. The p orbital overlaps with the ring π system extending the system to include the extra electron pair.
This addition of electrons to the ring π system is called ring activation. The carbon atoms at positions 2, 4 and 6 are given enhanced electron density and become activated towards electrophiles such as the bromonium ion Br+ or the nitronium ion NO2+.
The resultant effect is that much milder conditions are required to brominate phenol i.e. no halogen carrier and simply the addition of bromine water. The resultant white precipitate is the 2,4,6-tribromophenol. (see the equation below).
The -OH group then is said to be 2,4,6 directing.
Phenol with nitric acid
In much the same as with bromine water, phenol also reacts under very mild conditions with nitric acid. Unlike in the case of benzene where concentrated acid was required in the presence of concentrated sulphuric acid, the nitration of phenol takes place in the presence of just dilute nitric acid.
Two products usually result at room temperature 2-nitrophenol and 4-nitrophenol.
They can be separated since the molecules have different boiling points.
Again we see the 2,4,6 directing effect of the OH group in phenol since these positions on the ring are given enhanced electron density and make the ring more susceptible to electrophilic substitution.
The effect of other groups on the benzene ring is to do the same as phenol so for example the amine group NH2 is also 2,4,6 directing towards electrophiles as it too adds an electron lone pair from a nitrogen p orbital to the ring π system and makes the ring more susceptible to electrophilic substitution.
Bromination and nitration of phenylamine happen under the same kind of mild conditions as they do in phenol. (see equation below)
However if the substituent on the benzene ring is not electron pushing but electron withdrawing ( like a nitro group NO2) then the opposite effect on electrophiles is observed.
So for example the nitration of nitrobenzene (at temperature greater than 55oC ) produces 1,3–dinitrobenzene because the nitro group is 3,5 directing as it deactivates the ring towards electrophilic substitution.















No comments:
Post a Comment