In this blog we’ll discuss the water solubility of carboxylic acids, their dimerisation, attempt to account for their weakness and look at the structure of the carboxylate anion as another example of the delocalisation of electrons.
The simplest carboxylic acid is formic or methanoic acid.
It used to be called formic acid and still is by many chemists.
The word formic reminds us of the origins of this acid: it is the acid found in an ant sting–the Latin for ant being Formica.
The red ants of course also contained the red pigment cochineal and they when crushed produced a beautiful red paste that looked great on lips – the first type of lipstick.
Women in the later Middle Ages and early modern period used this stuff but the effect on the lips of the acidic contents led to infection and probably lip erosion! Ugh!
You can read more about the early history of lip stick here.
Here is the structural formula of formic acid: HCOOH and its displayed formula is this:
Solubility of Carboxylic Acids in Water
The structure of the carboxyl group means it is fully miscible with water with up to four carbon atoms in the carbon chain.
Only as the carbon chain increases considerable in size does the acid become insoluble e.g. when the carbons chain contains 8+ carbon atoms.
So for example benzoic acid with a bulky ring of 6 carbon atoms attached to the carboxyl group is soluble in hot water.
With a bulky group of carbon atoms attached to the carboxyl group energy is released when the group hydrogen bonds with water.
However, this energy is insufficient to break the stronger van der Waals forces that hold the hydrophobic carbon groups together so the acid is insoluble in water.
Let’s say that the solubility of these acids in water is due in the main to hydrogen bonding with water, as in the diagram below:
There are two other considerations for us when looking at solubility in water.
Dimerisation of Carboxylic Acids
First the lower molecular mass acids dimerise in water.
You can see how they do that in this diagram below:
Each acid hydrogen bonds to the other.
Because of dimerisation the molecular mass of a carboxylic acid is often measured to be twice its actual value.
Reaction with water: acidic properties
Second, the acids actually react with water that’s why they are acids after all and release H+ ions.
So CH3COOH + H2O ⇌ CH3COO— + H3O+
Question is why are all these acids weak acids and not strong acids?
Why is the dissociation of the ethanoic acid into the ethanoate ion and a hydrated proton only partial and incomplete?
To answer this question we need to look at the structure of the ethanoate ion itself.
Here are some representations of this anion:
The first image below shows the two resonance hybrids of the ethanoate ion in which the negative charge on one oxygen is envisaged as oscillating between the two oxygen atoms in the anion.
In the next image below, we can see from the reaction that the dissociation happens when water abstracts a proton from the ethanoic acid leading to the formation of the ethanoate anion and an oxonium ion H3O+.
It is this reaction that is energetically difficult so that the dissociation is only partial and therefore the acid is weak.
So why is benzoic acid stronger than ethanoic acid?
Consider this argument:
If X-COOH and Y-COOH are two carboxylic acids.
1 If X is more electronegative and electron-pulling than Y then X—COOH will be a stronger acid than Y—COOH. (Note: X or Y can be hydrogen).
2 Carbon shows positive inductive (+I) effect/is less electron pulling than hydrogen. So hydrogen is more electronegative compared to carbon.
3 sp2 hybridised carbon shows weaker +I effect/is more electron–pulling compared to sp3 carbon. Hence the phenyl group (C6H5—) is more electronegative than the methyl group (CH3) but still less electronegative than hydrogen.
If the electronegativity order of the substituents is H—>C6H5—>CH3—, then the order of acid strength is HCOOH > C6H5COOH > CH3COOH.
Consider the case of trichloroethanoic acid Cl3CCOOH, the three chlorine atoms are very electronegative and electron–pulling and draw the electrons from the –O—H bond.
Thus the –O—H bond is weakened before it even dissociates and this makes its dissociation easier and the acid stronger than ethanoic acid.
This is the key consequence of the structural difference between carboxylic acids.
It is for these structural reasons that the Ka and pKa values of the carboxylic acids discussed are as follows:
pKa (at 298K)
Trichloroethanoic acid 0.7
Formic acid 3.8
Benzoic acid 4.2
Ethanoic acid 4.8
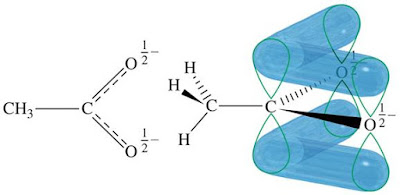
Structure of the Carboxylate Anion (—COO—)
In the next image we can see a molecular orbital picture of the ethanoate anion.
There is a π electron cloud above and below the plane of the ethanoate ion.
The effect of this distribution of the negative charge on the anion is to delocalise it.
The next image brings together the two ideas of the resonance hybrid and the delocalised electron in a molecular orbital:
















No comments:
Post a Comment