Ionic Bonding (2) Representing ionic
bonds with dot-cross diagrams (Lewis structures)
So here we
are again trying to give a few words of wisdom about ionic bonds.
Let’s start
by being controversial and say that the ionic bond does not exist in its raw
form.
Unlike say
hydrogen gas where we can see a discrete covalent bond of two electrons
perfectly shared between two atoms you don’t get that sort of thing with ionic
bonding.
Ionic bonds
exist in massive clusters called structures.
An ionic
bond never occurs in isolation.
Rather its
better to talk about moles of ionic bonds.
Which
reminds me to mention in the blogs I’m going to write for the next few weeks
that Mole Day will be upon us soon.
Nevertheless
you are going to find the ionic bond spoken about pretty frequently.
You’ll have
been taught that its formed by the donation of electrons from a metal atom to a
non metal atom.
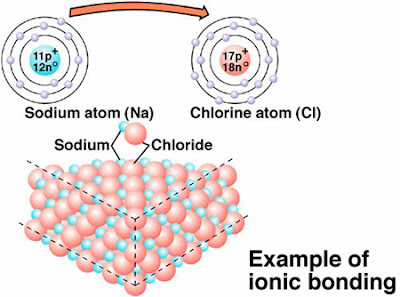
Here’s a typical diagram illustrating this
process which you can find everywhere on the Internet:
Here’s your
first diagram that shows why you
shouldn’t believe the Internet ever.
All is going
fine with this diagram until we get to the second line “to form a molecule of sodium chloride”
No, 1000 times no!!!! someone has to
speak out here at this …p.
I suggest
you try that line (about the molecule!) on your chemistry tutor and see what
their reaction is!!
I would
annihilate you actually if it were me.
And here is
another dodgy diagram:
I just don’t
like the red splodge labelled ionic bond.
It smacks
too much of a covalent bond picture like an σ bonding molecular orbital.
Although
this next diagram doesn’t give enough information at least it gives us the
accompanying structure of the ionic compound formed.
But let’s have the sodium labelled as
an “Ion” and same with the chloride it’s also an “ion”.
The colours
are immaterial.
There’s no
colour at the atomic level.
But the
structure is correct for sodium chloride as its has 6:6 coordination.
What that
means is that around each sodium ion are
6 chloride ions and vice versa.
So you don’t
just get one positive ion attached to one negative ion.
The formula
of an ionic compound just gives you the overall
ratio of the numbers of positive to negative ions.
The stoichiometric ratio…..
That’s 1:1
in the case of sodium chloride hence NaCl.
And as the
ratio is 1:1, there are no subscript stoichiometric numbers required.
What you
find with these diagrams is that they don’t show ionic bonds but the process of
how they are formed.
So you have
the transfer of outer shell electrons
from the Group 1, 2 or 3 element (usually a metal) to the outer shell of the
Group 5, 6 or 7 element in order to fill the outer shell with sufficient
electrons.
Here is a
classic diagram you have probably seen more times than sliced bread.
Now the
reason I like this diagram is not for the pink and pale blue blobs that are
supposed to represent electrons.
Nor the
distinguishing dots and crosses. Very
sad!!
Nor the correct numbers of electrons in each shell of
the atoms and the ions, good one that!!
But I like the brackets (or parentheses for some of you)
that encase the ion arrangements with the appropriate charge outside the
bracket.
I also like the electron in the chloride
in outer shell keeping its original colour and cross because that’s what
examiners like to see.
I also like
the statement about 1 electron transferred.
There’s none of this nonsense about
atoms “wanting” electrons
to fill their outer shells.
Atoms are
inanimate objects without personality or personal traits so they can’t want
anything. Its crazy!!!
You really
need to watch your use of language
in this topic.
But best of
all is the comment in the middle of the diagram between the ions.
Here the
attraction between ions is labelled correctly with a word not well known or
remembered by students of chemistry at school or college level.
The
attraction between ions is termed electrostatic
attraction. Yes!!!
Now, we like
this!!
We can also
see that each ion has a Noble gas
electron configuration once it has formed.
Sodium that
of Neon and the chloride ion that of Argon.
So finally,
let me challenge you to draw up a few of these dot and cross diagrams or Lewis
structures to illustrate the ionic bonding in some simple binary ionic
compounds like magnesium chloride, potassium oxide and lithium fluoride.
Some humorous diagrams from the Internet with the odd sexist joke thrown in.
More on
Ionic bonding in the next blog especially on the Born Haber Cycle.