It certainly does get very interesting when propene is used instead of ethene to illustrate the electrophilic addition across the double bond using bromine in water.
What difference could propene possibly make?
Let's recap for a minute.
What happens when bromine reacts with water?
Br2 + H2O = HBr + HOBr
Two compounds form: hydrobromic acid and bromic(I)acid.
Both these compounds are polar and could therefore engage in the electrophilic addition mechanism for the bromination of propene.
With bromine and with water the example in the previous post (Hydrocarbons(11)) of ethene and bromine water is followed.
Here is the equation:
1,2-dibromopropene CH3CHBrCH2Br is formed together with the bromohydrin CH3CHBrCH2OH
The interesting case is that of hydrobromic acid (HBr) or hydrogen bromide.
HBr is already a polar molecule due to the differences in electronegativity between hydrogen (2.2 on the Pauling Scale) and bromine (2.96).
δ+ δ-
We can write hydrobromic acid as H—Br.
When HBr adds across the propene double bond, it adds via the more stable carbocation.
This is referred to as Markovnikov's Rule.
To quote ROC Norman: "The underlying principle is that the electron releasing alkyl groups stabilise carbocations more effectively when they are bound directly to the positively charged carbon than when they are further removed from it."
So the primary carbocation:
is less stable than the secondary carbocation:
The electron releasing alkyl groups are the CH3– methyl groups and ethyl group (CH3CH2–) attached to the C+ atom.
There is only one of these in the primary propene carbocation but two alkyl groups in the secondary carbocation.
Therefore the secondary carbocation will be formed faster by the addition of a proton to propene.
Here is the electrophilic addition of the hydrogen bromide to propene:
Here then is summary of the energetic stability of carbocations (once called carbonium ions)
R— stands for an alkyl group and R' or R'' are different types of alkyl group.
The energetic stability increases left to right in the table.
Here is another diagram to illustrate that the increased inductive or
electron pushing effect of the alkyl groups stabilises the carbocation:

In this diagram above energetic stability increases right to left.
The arrow on the bond is a way of showing the inductive or electron pushing effect of the alkyl groups.
Pages on the "Mole" and "Using the Mole" in chemical calculations are here
What difference could propene possibly make?
Let's recap for a minute.
What happens when bromine reacts with water?
Br2 + H2O = HBr + HOBr
Two compounds form: hydrobromic acid and bromic(I)acid.
Both these compounds are polar and could therefore engage in the electrophilic addition mechanism for the bromination of propene.
With bromine and with water the example in the previous post (Hydrocarbons(11)) of ethene and bromine water is followed.
Here is the equation:
1,2-dibromopropene CH3CHBrCH2Br is formed together with the bromohydrin CH3CHBrCH2OH
The interesting case is that of hydrobromic acid (HBr) or hydrogen bromide.
HBr is already a polar molecule due to the differences in electronegativity between hydrogen (2.2 on the Pauling Scale) and bromine (2.96).
δ+ δ-
We can write hydrobromic acid as H—Br.
When HBr adds across the propene double bond, it adds via the more stable carbocation.
This is referred to as Markovnikov's Rule.
To quote ROC Norman: "The underlying principle is that the electron releasing alkyl groups stabilise carbocations more effectively when they are bound directly to the positively charged carbon than when they are further removed from it."
So the primary carbocation:
is less stable than the secondary carbocation:
The electron releasing alkyl groups are the CH3– methyl groups and ethyl group (CH3CH2–) attached to the C+ atom.
There is only one of these in the primary propene carbocation but two alkyl groups in the secondary carbocation.
Therefore the secondary carbocation will be formed faster by the addition of a proton to propene.
Here is the electrophilic addition of the hydrogen bromide to propene:
Here then is summary of the energetic stability of carbocations (once called carbonium ions)
R— stands for an alkyl group and R' or R'' are different types of alkyl group.
The energetic stability increases left to right in the table.
Here is another diagram to illustrate that the increased inductive or
electron pushing effect of the alkyl groups stabilises the carbocation:

In this diagram above energetic stability increases right to left.
The arrow on the bond is a way of showing the inductive or electron pushing effect of the alkyl groups.
Pages on the "Mole" and "Using the Mole" in chemical calculations are here
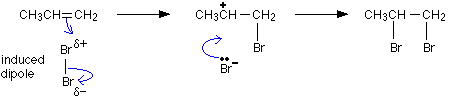













No comments:
Post a Comment