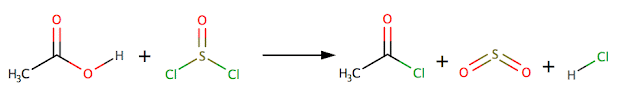In
this new post, I’m discussing Acyl Chlorides.
You’ll
probably have come across them as Ethanoyl Chloride or Benzoyl Chloride
What
you might not know however is that molecules like these are major constituents
of tear gas.
Protestor
catches Tear Gas canister in Tahir Square, Cairo
Exploding
tear gas canister on the fly!!
CS
and CN gas (or Mace) contain the following molecules:
CS
gas
Tear
gas was first used when the French fired canisters into German trenches at the
start of WW1
Tear
gas and acyl chlorides are examples of molecules called lachrimators
Molecules in tear gas and molecules like them causes burning of the eyes
and skin, tears, and a sensation of suffocation.
Their use is aimed at driving people from their cover and disrupting their
protest.
The gas also provokes fear and insecurity playing on the human survival
instinct.
It has been claimed that it is easier for men to face bullets than
invisible debilitating gas.
You
can read more about tear
gas here.
Preparation of acyl
chlorides
Acyl
chlorides can be prepared from the corresponding carboxylic acid using a
suitable chlorinating agent e.g. PCl5 phosphorus pentachloride or
SOCl2 thionyl chloride
Or
The
formation of this intermediate then enables the formation of many different
carboxylic derivatives in very high yields and atom economies.
So
for example:
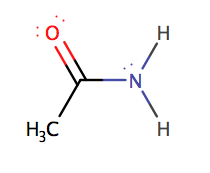
Ethanoyl
chloride and ammonia produce acetamide (ethanamide) a quick reaction at room
temperature
Ethanoyl
chloride and phenylamine produce N-phenylethanamide, an anti-coagulant.
Ethanoyl
chloride and ethanol (or any carboxylic acid and any alcohol) form esters at
high yield.
Ethanoyl
chloride is easily hydrolysed using water to form the corresponding carboxylic
acid.
Summary of
carboxylic acid derivative chemistry
“Unsaturated
carbon in a suitable environment undergoes substitution via a two step process consisting of addition followed by
elimination.” ROC Norman
Substitution
at a carbonyl group involves a nucleophile.
Carboxylic
acid derivatives where the carbonyl group is attached and adjacent to an
electronegative atom are substituted via an addition–elimination mechanism.
Among
the common carboxylic derivatives the order of reactivity is acid chloride >
acid anhydride > ester > amide.
Acid
chlorides react rapidly with water, anhydrides slowly and esters and amides not
at all.
Here
is a typical nucleophilic substitution mechanism: