GCSE OCR Gateway Organic
Chemistry C6.2i
Summary
Common misconceptions
Carbon chemistry is the basis
of life on Earth. Organic chemistry is the basis of many of the materials we
produce. Organic compounds are covalent in nature and react in a predictable
pattern. Crude oil forms the basis of many useful by-products.
CM6.2i To be able to represent
three-dimensional shapes in two dimensions and vice versa when looking at
chemical structures, e.g. allotropes of carbon.
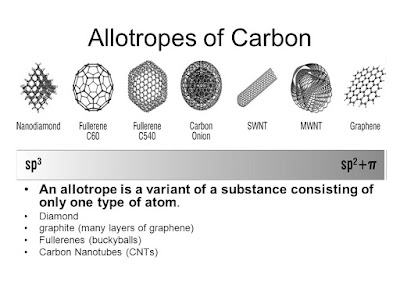
Allotropes
of carbon
Allotropes are different
structural forms of the same element in the same physical state.
Carbon has several allotropes
in its solid state: Diamond, Graphite , graphene, fullerenes and nanotubes.
Diamonds
Graphite
Buckminsterfullerene C60 (dissolved
in benzene)
flakes of graphene welded
together
Allotropes of carbon are based
on the ability of carbon to demonstrate three dimensionality.
Each carbon atom can form up
to four covalent bonds with itself or with other elements.
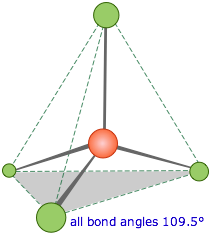
When carbon forms four
covalent bonds with itself, it forms a diamond structure. Each bond points to the corner of a regular
tetrahedron as in the diagram below.
The covalent
bonds are very strong they require massive amounts of energy to break them all
and so when combined in the giant diamond structure give diamond its incredible
hardness and strength.
Diamond is
the hardest known material. Hence it is used in drill bits. It will easily cut glass.
As all the
outer shell electrons of the carbon atoms are used in the four bonds there are
no delocalised electrons to allow for the conduction of electricity so carbon
as diamond is an insulator.
Breaking
these strong covalent bonds as I have said requires massive amounts of energy
and so the material is insoluble in water.
On the other
hand carbon atoms in graphite are bonded differently. Unlike in carbon there are only three bonds
per carbon atom n graphite.
Each bond is
a very strong covalent bond but as there are only three per carbon atom then
the carbon atoms are arranged differently to diamond.
In graphite
the carbon atoms are arranged in layers.
The diagram below shows how this happens:
As a result
each carbon atom has a delocalised electron to contribute to the conduction of
electricity in graphite. Graphite is
good conductor of electricity.
The layers
repel each other too and are loosely bonded to each other so that they are able
to slide over each other. Hence,
graphite is a smooth and slippery substance and is used in lubricants. For the same reason, it is also found in
pencil “lead” where it is baked with varying amounts of clay to give different
degrees of softness to the “lead”.
Graphene is
related to graphite since it is composed of a single layer of carbon atoms like
those found in graphite.
Fullerenes
on the other hand and single wall nano tubes are formed as if the grapheme layer
of carbon atoms had been rolled into a tube (SWNT) or folded up into a ball
(fulllerenes)
You can see
this from the diagram below:
This final
chart summarises the properties of the carbon allotropes