C6.1q To be able to explain
how mitigation of corrosion is achieved by creating a physical barrier to
oxygen and water and by sacrificial protection
How to stop iron rusting
Protective methods
One obvious
way of protecting iron and steel from rusting is to coat the metal with a
material that water and air cannot penetrate.
Traditionally
paint such as Hammerite has been used fairly successfully to protect iron from
rusting.
Similarly, oiling the iron
gives a more flexible coating that water cannot penetrate.
These traditional methods work but are not
suitable for all conditions particularly when iron is exposed to sea water a
much more vigorous and corrosive environment.
So how can
iron and steel be protected from corrosion especially since most boats and
ships are steel hulled?
The answer
is in what’s called sacrificial protection.
Sacrificial methods
Because
corrosion is an oxidative process connecting iron to a more reactive metal will
protect it from rusting.
What happens
is that the more reactive metal will oxidise instead of the iron it protects
Typically,
magnesium and zinc are used to protect iron .
Its called sacrificial since the other more reactive metal is eventually
consumed and sacrificed to protect the iron,
You can see
blocks of magnesium bolted to ships hull for this very purpose
You can also
set up an interesting and colourful experiment to show sacrificial protection
happening.
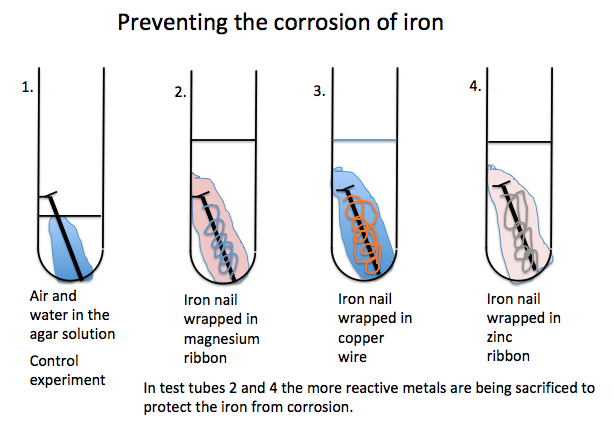
You need to
make up a hot agar solution containing traces of both potassium hexacyano
ferrate(III) (K3Fe(CN)6)
and the acid base indicator phenolphthalein.
Pour this
solution into test tubes that contain iron nails, one on its own, another
wrapped in copper wire, another wrapped in zinc plate and a fourth wrapped in
magnesium ribbon.
Here is the
set up:
And in
photo:
The blue
colour is due to the formation of Prussian Blue a distinctive blue colour that
shows the presence of iron (III) (Fe3+) ions in rust.
The magenta
colour shows how the magnesium or other reactive metal is protecting iron from
corrosion. The magnesium has reacted to
form an alkaline solution hence the phenolphthalein has turned magenta.
The next
photo shows how a boat hull is being protected from corrosion using blocks of
magnesium or zinc bolted to the hull.
The other photo shows a corroded zinc
block on a ships hull.














No comments:
Post a Comment